Question
Question: Vinegar is a dilute aqueous solution of which acid....
Vinegar is a dilute aqueous solution of which acid.
Solution
Dilute acetic acid solution is called vinegar. The IUPAC name of acetic acid is ethanoic acid which contains carboxylic acid as a functional group. Acids are sour in taste and have a pungent smell.
Complete answer:
Vinegar is a dilute aqueous solution of acetic acid. As vinegar contains acid and thus it is sour in taste and has a pungent smell. Approximate 5 % of acetic acid is added into 95 % of water to make vinegar. The chemical formula of acetic acid is CH3COOH and its structure is given as follows:
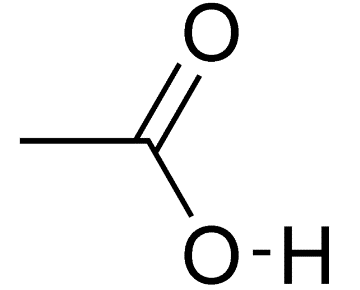
From the above structure, we conclude that carboxylic acid is present as a functional group in acetic acid. The IUPAC name of acetic acid is ethanoic acid.
Additional Information:
The word “Vinegar” is derived from latin in which “vin” refers to wine and “egar” refers to sour. Vinegar is commercially synthesized by the fermentation of sugar in the presence of yeast. Vinegar has antibacterial properties and that’s why it is used as a preservative in the kitchen in order to inhibit food spoilage. For instance, vinegar is used as a preservative in making pickles. Moreover, this antibacterial property of vinegar is also utilized in farms to inhibit bacterial and fungal growth.
Note:
It is important to note that vinegar is a dilute aqueous solution of acetic acid or ethanoic acid. Approximate 5 % of acetic acid is added into 95 % of water to make vinegar. Vinegar has a wide range of applications in everyday life especially in cooking, baking, in making pickles, in cleaning and so on.
