Question
Question: (vii) Which formula co-relates degree of dissociation and concentration of electrolyte?...
(vii) Which formula co-relates degree of dissociation and concentration of electrolyte?
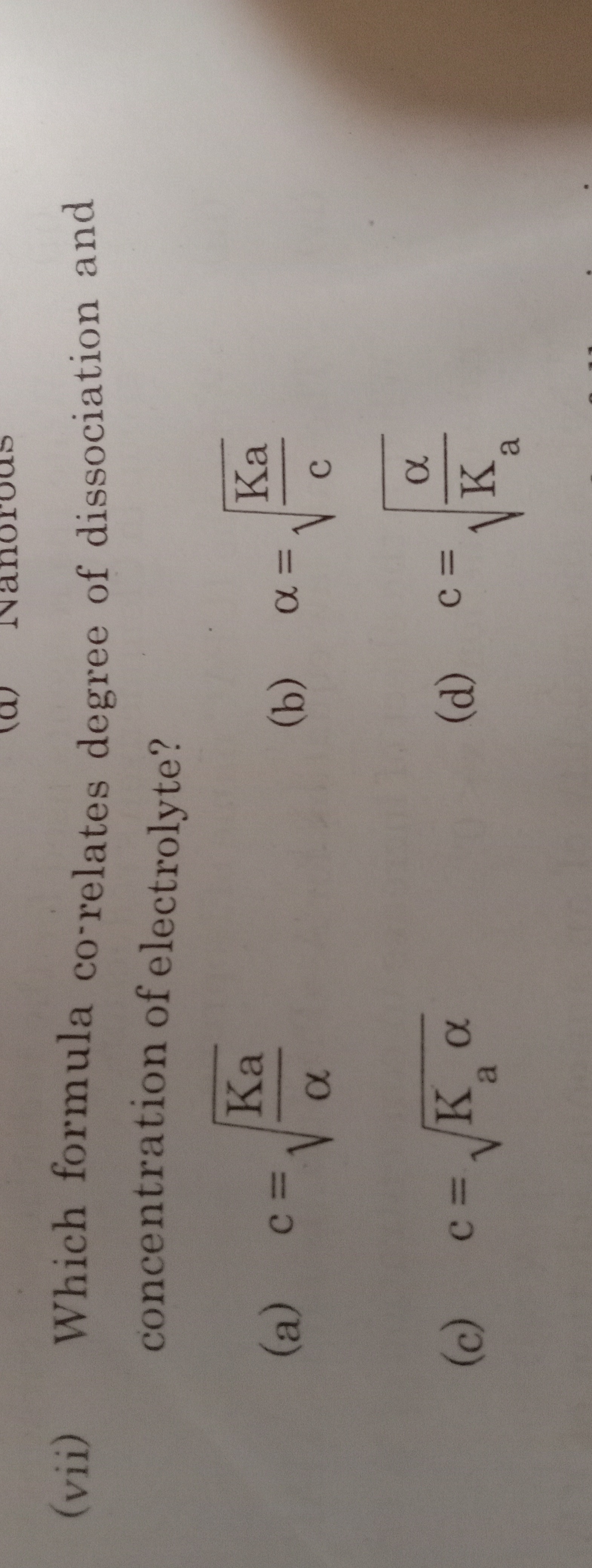
A
c=αKa
B
α=cKa
C
c=Kaα
D
c=Kaα
Answer
α=cKa
Explanation
Solution
The degree of dissociation (α) of a weak electrolyte is related to the acid dissociation constant (Ka) and the concentration (c) by the formula:
α=cKa
This relationship is derived from Ostwald's Dilution Law, which simplifies under the assumption that α is very small compared to 1.
