Question
Question: Vander Waal's gas equation is: $(P + \frac{a}{V^2})(V-b) = RT$. The dimensions of constant a as give...
Vander Waal's gas equation is: (P+V2a)(V−b)=RT. The dimensions of constant a as given above are:
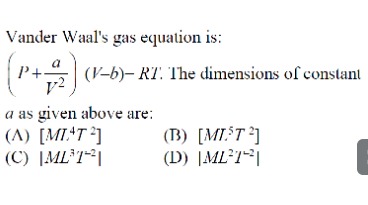
A
[ML4T−2]
B
[ML5T−2]
C
[ML3T−2]
D
[ML2T−2]
Answer
(B) [ML5T−2]
Explanation
Solution
The principle of dimensional homogeneity states that terms added or subtracted in an equation must have the same dimensions. In the Van der Waals equation, (P+V2a), the term V2a is added to pressure P. Therefore, the dimensions of V2a must be equal to the dimensions of pressure P.
Dimensions of Pressure (P) are [ML−1T−2].
Dimensions of Volume (V) are [L3].
Thus, [V2a]=[P] implies [L3]2[a]=[ML−1T−2].
Solving for [a]: [a]=[ML−1T−2]×[L6]=[ML5T−2].
