Question
Question: Van Arkel method of purification of metals involves converting the metal to a: A. Volatile compoun...
Van Arkel method of purification of metals involves converting the metal to a:
A. Volatile compound
B. Volatile unstable compound
C. Non-volatile unstable compound
D. None of these.
Solution
Try to recall that Van Arkel is used for refining of metals and by this method, you will get ultra pure metal. Also, this method is based on the thermal decomposition of metal components. Now, by using this you can easily find the correct option from the given options.
Complete step by step answer:
- We know that this method is also known as vapour phase refining.
- This method is based on the fact that certain metals are converted to their volatile compounds while impurities are not affected during compound formation.
- Volatile compounds are compounds that have a relatively higher than normal vapour pressure at room temperature, these compounds are the most likely to easily escape into the air as a gas at the slightest energy provided. Some examples of volatile compounds are benzene, toluene, formaldehyde, alcohols, etc.
- Van Arkel method is used for getting ultra pure metal. In this method, metal is converted to volatile unstable compounds taking care that impurities are not affected.
- The volatile unstable compound thus obtained is decomposed to get the pure metal.
- This method is used for the purification of metals like titanium and zirconium.
- In impure metals like M=Ti or Zr, metal is heated at 2500 degree Celsius in an evacuated vessel with iodine. MI4 is formed which evaporates leaving behind impurities. The gaseous MI4 decomposes on a white tungsten filament and the impurities are left behind as they do not react with iodine.
- Some reactions that involve titanium and zirconium going through this process are:
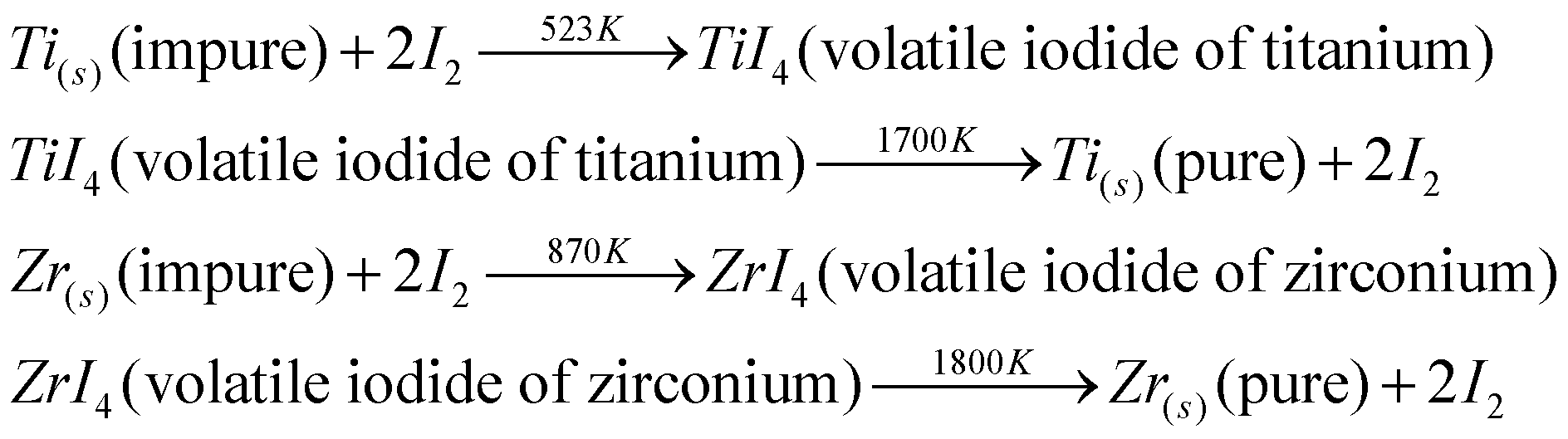
So, the correct answer is “Option B”.
Note: Note that in this method, the metal should form a volatile compound with available reagent. Also, you should remember that the volatile compound formed should be easily decomposable so that the metal can be easily recovered.
