Question
Question: Value of equilibrium constant for following reaction, N$_2$(g) + 3H$_2$(g) $\rightarrow$ 2NH$_3$, (g...
Value of equilibrium constant for following reaction, N2(g) + 3H2(g) → 2NH3, (g) is 0.50 at 400°C. What is the value of Kp at 400°C ?
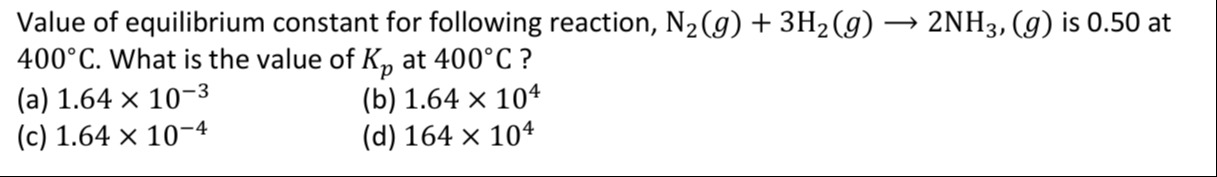
A
1.64 × 10−3
B
1.64 × 104
C
1.64 × 10−4
D
164 × 104
Answer
1.64 × 10−4
Explanation
Solution
The equilibrium constant Kp is related to Kc by the equation Kp=Kc(RT)Δng.
For the reaction N2(g) + 3H2(g) → 2NH3(g), Δng=2−(1+3)=−2.
The temperature is T=400∘C=400+273=673 K.
Using R=0.0821 L atm/mol K and Kc=0.50, we calculate Kp.
Kp=0.50×(0.0821×673)−2=0.50×(55.2533)−2=0.50×3053.021≈0.50×3.275×10−4≈1.6375×10−4.
This value is approximately 1.64×10−4.
