Question
Question: \(V\) versus \(T\) curves at constant pressure \({P_1}\,and\,{P_2}\) for an ideal gas are shown in t...
V versus T curves at constant pressure P1andP2 for an ideal gas are shown in the figure below. Which is constant?
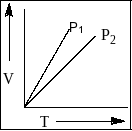
(A) P1>P2
(B) P1<P2
(C) P1=P2
(D) none of the above
Solution
For ideal gases the pressure, temperature and volume are related to each other by different laws. These laws combine to give one common law known as combined gas law. One can relate these terms with each other in the equation and find out the value which is constant.
Complete step by step answer:
- First of all we will learn about the combined gas law where we can relate the terms with each other, it can be defined as for a sample of gas, the ratio of the product of the original pressure and volume to the original temperature is constant. It can be written in mathematical formula as,
TPV=K,
Where, P is pressure, V is volume, T is temperature and K is constant.
The above equation can also be written as, TV=PK - It can be stated from the above mathematical equation that the ratio of volume and temperature is inversely proportional to the pressure. Thus, in a volume versus temperature graph the slope of the graph is inversely proportional to the pressure of the gas.
- It can be said that the pressure is more if the slope of volume versus temperature graph is less and pressure is less if the slope of volume versus temperature graph is more.
In the given graph slope of P1>P2 . So, the value of P2>P1 .
Therefore, P2>P1 which shows option ‘B’ as a correct choice.
Note:
The ideal gas law is also known as the general gas equation. All the real gases behave as ideal gas at very low pressure and high temperature. The inversely proportional value should be analyzed with relevant terms and necessary changes should be made in equations like changing the sides. The given slope of the graphs should be observed carefully.
