Question
Question: Using Valence Bond theory, Account for the geometry and magnetic property of \({\left[ {{\mathbf{Ni}...
Using Valence Bond theory, Account for the geometry and magnetic property of [Ni(CN)4]2−
Solution
Many theories have been proposed to clarify the existence of coordination compound bonding. The Valence Bond (VB) Theory is one of them. The Valence Bond Theory was created in order to use quantum mechanics to describe chemical bonding. During the formation of a molecule, this principle mainly focuses on the formation of individual bonds from the atomic orbitals of the involved atoms.
Complete answer: Main assumptions of Valence Bond theory:
A coordination complex's ligand metal bond is covalent in nature. It is formed by the central metal atom and the ligand exchanging electrons (provided by the ligands). At least one filled orbital containing a lone pair of electrons should be present in each ligand.
The central metal ion in a complex supplies the necessary number (coordination number) of empty orbitals to satisfy the electron pairs donated by the ligands.
Hybridisation, or the mechanism of combining, occurs in these empty orbitals of the central metal atom.
The formation of an equivalent number of new orbitals from atomic orbitals of comparable energy, orbitals that have been hybridised and have the same capacity.
The central metal ion's empty hybridised orbitals line up with the ligands' filled orbitals to form coordinate covalent sigma bonds between the metal and the ligand.
The hybridised orbitals are directional, and their spatial orientation gives the complex ion a specified geometry.
Inner orbital complexes, low spin complexes, or spin paired complexes are octahedral complexes in which the (n-1) d orbitals are involved in hybridisation. These complexes are known as outer orbital, high spin, or spin free complexes where the nd orbitals are involved in hybridisation. The principal quantum number of the outermost shell is defined by n.
Paramagnetic complexes contain a core metal atom with unpaired electron(s). The complexes would be diamagnetic if any of the electrons are combined.
[Ni(CN)4]2−
Central metal atom/ion and its outer electronic configuration = Ni2+:3d8,4s0
Outer orbitals of metal atom/ion = 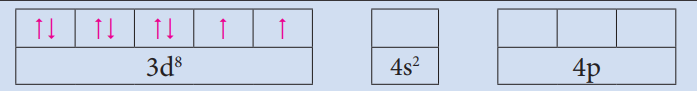
Nature of ligand = CN−
Strong field ligand causes the pairing of 3d electrons in the metal
Outer orbitals of metal atom/ion in presence of ligands =
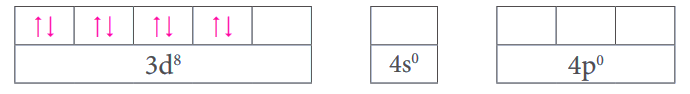
Coordination number - 4
Hybridisation - dsp2
Hybridised orbitals of the metal atom in the complex=
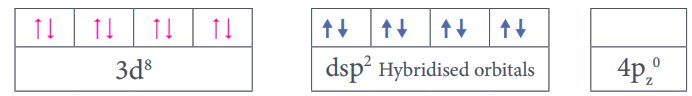
Geometry = Square planar
Magnetic property = Diamagnetic
(Since Number of unpaired electrons is zero)
Magnetic moment (Using spin only formula) = ∝s=n(n+2)=0
Note:
The valence bond theory has a number of flaws.
The inability to demonstrate the tetravalency of carbon.
The energies of the electrons are not discussed.
Electrons are said to be clustered in small locations, according to the hypothesis.
It does not have a quantitative interpretation of coordination compounds' thermodynamic or kinetic stabilities.
There is no difference between weak and heavy ligands.
There is no reason for the colour of coordination compounds.
