Question
Question: Using the Hess’ Law, how to calculate the standard heat of formation of copper(I) oxide given in the...
Using the Hess’ Law, how to calculate the standard heat of formation of copper(I) oxide given in the following data?
CuO(s)→Cu(s)+21O2ΔH=157.3kJ/mol
4CuO(s)→2Cu2O(s)+21O2(g)ΔH=292.0kJ/mol
Solution
The Hess’s law is major in physical chemistry which states that the total enthalpy change for a reaction is independent of the route by which the chemical change takes place. Formation of heat is defined as the enthalpy change which accompanies the formation of 1 mole of substance in a standard state from its elements also taken in standard state.
Complete step-by-step answer:
As we know that Hess law takes the independent route for the reaction. In thermodynamics, we need the initial and final states. So, we need to construct a Hess Cycle using the given information;
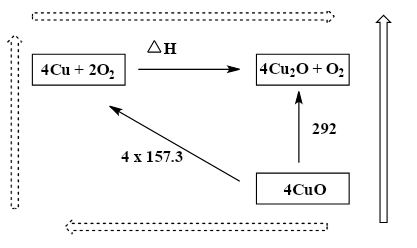
While we observe the diagram, in the terms of energy, the dashed long route is equal to the black arrow route as the arrows start and finish in the same place. This diagram represents and follows the Hess law.
So, we can write as; (4×157.3)+ΔH=292
Therefore, ΔH=−337.2kJ
Enthalpy of formation is defined as the enthalpy change which accompanies the formation of 1 mole of substance in a standard state from its elements also taken in standard state under the standard conditions.
We have foundΔHfor below equation;
4Cu+2O2→2Cu2O+O2
That means, it is the same as:
4Cu+O2→2Cu2O
This means the formation of 2 moles of copper(I) oxide.
So, we need to find the change in enthalpy for the formation of 1 mole of copper(I) oxide.
Therefore,
ΔHf[Cu2O]=2ΔH=−2337.2=−168.6kJ/mol
So, for the formation of 1 mole of copper(I) oxide we need −168.6kJ/mol enthalpy.
Note: We must know that Hess’s law is a version of the first law of thermodynamics. So, it means the energy is always conserved. Often Hess’s law cycles are used to measure the change in enthalpy for a reaction that can’t be measured directly by experiments. Instead, the alternative method/ reactions are carried out that can be measured experimentally.
