Question
Question: Using only a periodic table as a guide, how do you write the condensed electron configuration for th...
Using only a periodic table as a guide, how do you write the condensed electron configuration for the following atoms?
A.Rh
B.Si
C.Hg
D.Hf
Solution
The electronic configuration says about how the electrons are distributed in atomic orbitals. The electronic configuration is filled according to the energy they have. They follow the standard notion in which electrons containing the atomic subshells are placed in a sequence. For example, the Lithium’s electronic configuration; Li= 1s22s1.
Complete step by step answer:
The standard notation yields lengthy electronic configuration (which means the electronic configuration will be written as, ( Na= 1s22s22p63s1 ). In those cases, condensed or an abbreviated notation is used instead of using standard electronic configuration. In the condensed electron configuration, the sequence of filled subshell corresponds to the electronic configuration of a noble gas or inert gas is replaced with the symbol of that noble gas in a square bracket.
Therefore, the condensed electron configuration of sodium will be;
Na= [Ne]3s1
The full electronic configuration of any element is written in a standard format. But when it comes to higher elements it will consist of any number of orbits, so for simplification condensed electronic configuration is noted.
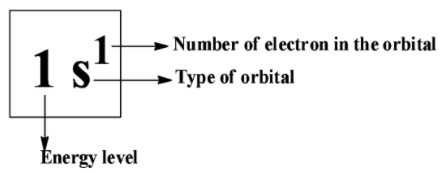
Suppose if we have considered iron as an example,
The full electronic configuration;
Fe= 1s22s22p63s23p64s23d6
The condensed electron configuration;
Fe= [Ar]4s23d6
Now, you are able to differentiate that a lengthy electronic configuration can be written in abbreviated electron configuration.
So, the condensed electron configuration for the following compounds are;
⇒Rh=[Kr]5s14d8
⇒Si=[Ne]3s23p2
⇒Hg=[Xe]6s24f145d10
⇒Hf=[Xe]6s24f145d2
Note: The electronic configuration follows the entire principles to how to fill the electrons. The principles are Aufbau’s principle, Hund’s rule and Pauli’s exclusion principle. Each of the rule say;
1. Aufbau’s principle: In its ground state, the electrons are filled in the atomic orbitals of an atom.
2. Hund’s rule: Each orbital of the subshell should be single filled with the electron then pairing takes place. All singly occupied electrons should have the same spin.
3. Pauli’s exclusion principle: It is impossible for two electrons or more electrons to have the same values of four quantum numbers.
