Question
Question: Using MOT, compare \(O_{2}^{+}\,and\,O_{2}^{-}\) species and choose the incorrect option. (A)- \(O...
Using MOT, compare O2+andO2− species and choose the incorrect option.
(A)- O2+ have higher bond order than O2−
(B)- O2−is less stable
(C)- O2+is diamagnetic while O2− is paramagnetic
(D)- Both O2+and O2− are paramagnetic
Solution
In the given species, using MOT we will determine the accommodation of the electrons in the molecular orbitals which will further help in determining the bond order, stability, and magnetic nature of the molecule.
Complete step by step solution:
In the oxygen atom, with Z = 8, it has electronic configuration 1s22s22p4. Then the total number of electrons in O2 is 16. In the O2+ form, deficient of one electron, it has a total of 15 electrons and with one extra electron the O2− form has 17 electrons.
The atoms combine together to form the molecule, in which the electrons are now accommodated in the molecular orbitals. So, the electronic configuration of the species is as follows:
O2+=(σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2(π2px)2=(π2py)2(π∗2px)1
O2+=(σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2(π2px)2=(π2py)2(π∗2px)2(π∗2py)1
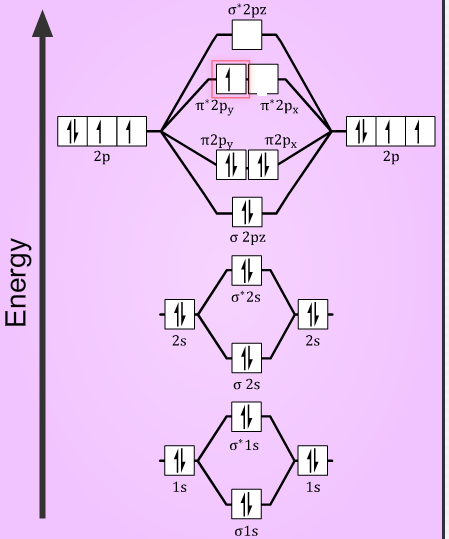
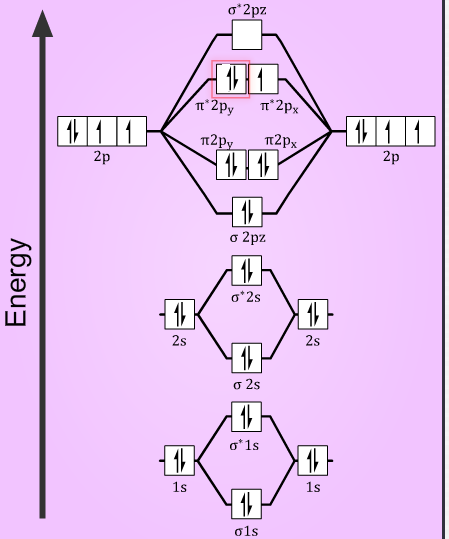
Then, the bond order of the species will be now determined using the number of electrons the bonding and the antibonding orbitals, from the formula:
Bondorder = 21 (no.ofelectronsinbondingorbitals - no.ofelectronsinanti-bondingorbitals)
So, in O2+ molecules with 10 electrons in bonding orbitals and 5 electrons in antibonding orbitals. The bond order is =2(10−5)=2.5
Similarly, in O2− molecules, with 10 electrons in bonding orbitals and 7 electrons in antibonding orbitals. The bond order is =2(10−7)=1.5
Thus, the bond order of O2+ is higher than O2− molecule.
Now, the stability of the molecule is determined by the number of electrons in the antibonding orbital. More the number of electrons in the antibonding orbitals, more is the instability of the molecule. So, the O2− with greater number of electrons in the antibonding orbitals compared to O2+ . It is less stable.
Also, with the number of unpaired electrons in the molecule, the magnetic nature can be determined. As from the electronic configuration it is seen that, both the species have one unpaired electron in their anti-bonding orbital. Thus, making them paramagnetic in nature.
Therefore, the incorrect statement for the given species will be option (C)- O2+is diamagnetic while O2− is paramagnetic.
Note: The energy of the bonding orbital is lower than the antibonding orbitals in the molecule. And the filling of electrons in the molecular orbitals also follows the Aufbau principle, Pauli’s exclusion principle and the Hund’s rule.
