Question
Question: Use the data below to calculate the standard enthalpy change of formation of Bi(CH3)3() in kJ mol¹ ...
Use the data below to calculate the standard enthalpy change of formation of Bi(CH3)3() in kJ mol¹
ΔcH∘[Bi(CH3)3(()]=−2912kJmol−1; ΔfH∘[CO2(g)]=−394kJmol−1
ΔfH∘[H2O(()]=−286kJmol−1; ΔfH∘[Bi2O3(s)]=−574kJmol−1
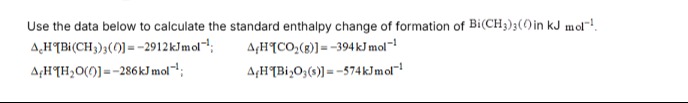
The standard enthalpy change of formation of Bi(CH₃)₃(l) is +156 kJ/mol.
Solution
-
Write the balanced combustion reaction for one mole of Bi(CH₃)₃. First, balance using 2 moles and then divide by 2:
2 Bi(CH₃)₃(l) + 12 O₂(g) → Bi₂O₃(s) + 6 CO₂(g) + 9 H₂O(l)
For one mole:
Bi(CH₃)₃(l) + 6 O₂(g) → ½ Bi₂O₃(s) + 3 CO₂(g) + (9/2) H₂O(l)
-
Use Hess's law with the combustion equation:
Δ_cH° = [0.5·Δ_fH°(Bi₂O₃) + 3·Δ_fH°(CO₂) + (9/2)·Δ_fH°(H₂O)] – Δ_fH°(Bi(CH₃)₃)
Given:
Δ_cH°[Bi(CH₃)₃] = –2912 kJ/mol
Δ_fH°(Bi₂O₃) = –574 kJ/mol
Δ_fH°(CO₂) = –394 kJ/mol
Δ_fH°(H₂O) = –286 kJ/mol -
Calculate the total enthalpy for products:
0.5·(–574) = –287 kJ
3·(–394) = –1182 kJ
(9/2)·(–286) = –4.5×286 = –1287 kJ
Sum = –287 – 1182 – 1287 = –2756 kJ -
Plug into the equation:
–2912 = (–2756) – Δ_fH°(Bi(CH₃)₃)
Solve for Δ_fH°(Bi(CH₃)₃):
Δ_fH°(Bi(CH₃)₃) = –2756 + 2912 = +156 kJ/mol
