Question
Question: Upon reaction with \[C{{H}_{3}}MgBr\]followed by protonation, the compound that produces ethanol is:...
Upon reaction with CH3MgBrfollowed by protonation, the compound that produces ethanol is:
(A) CH3CHO
(B) HCOOH
(C) HCHO
(D) (CHO)2
Solution
Alkyl magnesium halide (RMgX) is called Grignard reagent. The Grignard reagent reacts with carbonyl compounds and forms respective alcohols as the product. The reaction of the Grignard reagent with carbonyl compounds is an example of an additional reaction.
Complete step by step solution:
-The given Grignard reagent isCH3MgBr, methyl magnesium bromide.
-In the question, it is given that CH3MgBrreacts with one of the given options and forms ethanol as the product.
-Now we have to find the correct option to get ethanol when CH3MgBr reacts.
-Coming to the given options, option A, Acetaldehyde (CH3CHO).
CH3CHO+CH3MgBrH+CH3CH(OH)CH3
-Acetaldehyde reacts with methyl magnesium bromide and forms isopropyl alcohol as the product. So, option A is wrong.
-Coming to option B, HCOOH (formic acid). Option B is wrong because formic acid reacts with the Grignard reagent in tetrahydrofuran (THF) and forms aldehyde as the product, not ethanol.
HCOOH+CH3MgBrTHFCH3CHO
-Coming to option C, HCHO.
HCHO+CH3MgBrH+CH3CH2OH
-Formaldehyde (HCHO) reacts with CH3MgBrand forms ethanol as a product.
-Coming to option D, (CHO)2(Glyoxal). Glyoxal reacts with a given Grignard reagent and forms but-2,3-diol as the product.
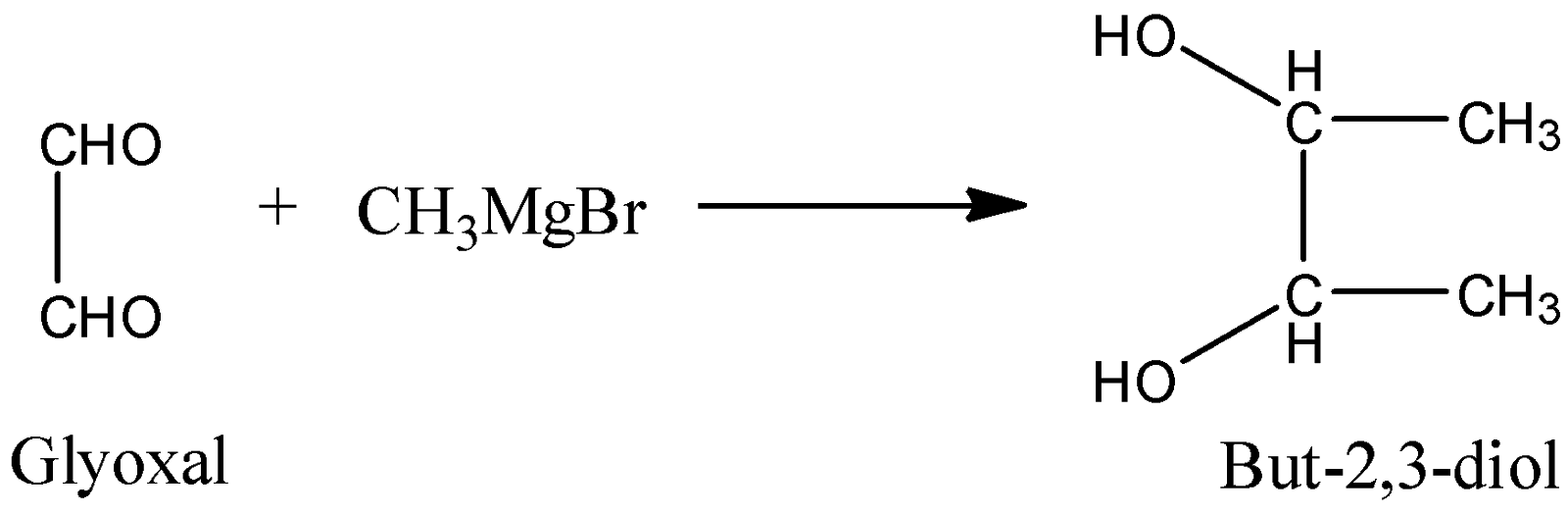
So, the correct option is (C), CH3MgBr reacts with formaldehyde and forms ethanol as the product.
Note: Grignard reagent reacts with ketone and forms tertiary alcohol as the product while Grignard reagent reacts with aldehydes and forms secondary or primary alcohols as the product. The reaction of excess amounts of the Grignard reagent with esters or with lactones produces tertiary alcohol as the product.
