Question
Question: Unlike phenol, 2,4-dinitrophenol is soluble in sodium carbonate solution in water because? A. pres...
Unlike phenol, 2,4-dinitrophenol is soluble in sodium carbonate solution in water because?
A. presence of two - NO2groups in the ring makes 2, 4 – dinitrophenol a stronger acid than phenol.
B. presence of two - NO2groups in the ring makes 2, 4 – dinitrophenol a weaker acid than phenol.
C. presence of two - NO2groups make the hydrogen bonding easier, making 2,4-dinitrophenol soluble.
D. nitro group reacts with Na2CO3while the -OH group does not.
Solution
To solve this question you should have a basic idea regarding the structures of phenol and 2,4-dinitrophenol and about electron withdrawing groups and its effect after adding to any compound. Phenol has a benzene ring with one hydroxyl group and 2,4-dinitrophenol has two nitrogen oxide molecules more than phenol.
Complete answer:
Structures of phenol and 2,4-dinitrophenol are drawn below.
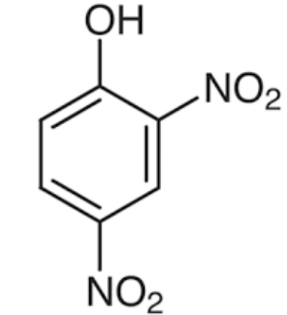
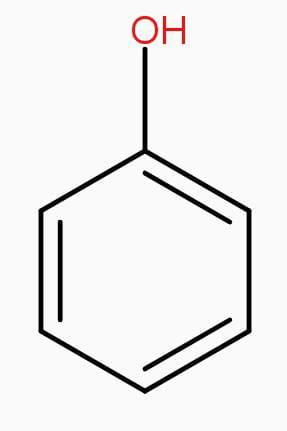
(2,4 – dinitrophenol) (phenol)
In 2,4 – dinitrophenol two - NO2 groups are present. NO2 has a strong electron withdrawing nature which increases acidity of the compound.
Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects.
Therefore, presence of two electron withdrawing - NO2groups in the ring makes 2,4- dinitrophenol a stronger acid than phenol.
Sodium Carbonate solution is basic in nature. Hence, Stronger the acid it is easily soluble in the basic solution.
Therefore, it reacts with Na2CO3solution to form sodium salt with the evolution of CO2, thus making it soluble inNa2CO3.
Hence, unlike phenol, 2,4-dinitrophenol is soluble in sodium carbonate solution in water because presence of two - NO2groups in the ring makes 2, 4 – dinitrophenol a stronger acid than phenol.
Hence, option A is the right answer.
Note:
Always remember, 2,4,6 – trinitrophenol > 2,4 – dinitrophenol > phenol, order of acidic nature of the compounds.
Orders of electron withdrawing group is -NO2, -CN,-CHO,-COR,-COOH,-COOR, -CONH2(strongest to weakest)
