Question
Question: Two soap bubbles A and B are kept in a closed chamber where the air is maintained at pressure \[8N/{...
Two soap bubbles A and B are kept in a closed chamber where the air is maintained at pressure 8N/m2. The radii of bubble A and B are 2 cm and 4 cm, respectively. Surface tension of the soap water used to make bubbles is 0.04 N/m. Find the ratio nAnB, where nA and nB are the number of moles of air in bubbles A and B, respectively. (Neglect the effect of gravity).
Solution
We are given the radii of two soap bubbles, the surface tension of the soap water used to make the bubbles and the pressure of the chamber where these bubbles are kept. By finding the pressure and volume of these two bubbles and substituting it in the ideal gas equation and then taking the ratio of these two equations we will get the required ratio.
Formula used:
P=P0+r4T
V=34πr3
PV=nRT
Complete answer:
In the question we are given two soap bubbles ‘A’ and ‘B’ which are placed in a closed chamber.
The pressure of the air in the chamber is 8N/m2. This is the external pressure, i.e.
P0=8N/m2
Surface tension of the soap water used to make the two soap bubbles is given as 0.04 N/m, i.e.
T=0.04N/m
Now let us consider the soap bubble ‘A’.
Let ‘rA’ be the radius of the soap bubble ‘A’, then we are given,
rA=2cm=2×10−2m
Let ‘PA’ be the pressure inside the soap bubble ‘A’.
We know that pressure inside is a bubble is given by the equation,
P=P0+r4T, were ‘P’ is the inside pressure, ‘P0’ is the outside pressure, ‘T’ is the surface tension and ‘r’ is the radius of the bubble.
Therefore the pressure inside the soap bubble ‘A’ is,
⇒PA=P0+rA4T
By substituting for the known values in the above equation,
⇒PA=8+2×10−24×0.04
By solving this we get,
⇒PA=16N/m2
Now we can calculate the volume of the soap bubble ‘A’.
The equation for volume of sphere is,
V=34πr3
Since soap bubble is sphere, the volume of soap bubble ‘A’ will be,
VA=34πrA3
By substituting for radius of the bubble ‘A’,
⇒VA=34π×(2×10−2)3
From the ideal gas equation, we know that
PV=nRT
Now let us find the ideal gas equation of the soap bubble ‘A’. it will be,
PAVA=nART
By substituting for pressure and volume, we get
⇒(16)(34π×(2×10−2)3)=nART
This is the ideal gas equation of soap bubble ‘A’.
Now let us consider the soap bubble ‘B’.
Let ‘rB’ be the radius of the bubble ‘B’. We are given,
rB=4cm=4×10−2m
Let ‘PB’ be the pressure inside the bubble ‘B’, then we know that,
PB=P0+rB4T
By substituting for the known values, we get
⇒PB=8+4×10−24×0.04
By solving this we get,
⇒PB=12N/m2
Now let us find the volume of the soap bubble ‘B’.
We know that,
VB=34πrB3
By substituting for radius of the bubble ‘B’, we get
⇒VB=34π(4×10−2)3
Now we know that the ideal gas equation for the soap bubble ‘B’ is given as,
PBVB=nBRT
By substituting for pressure and volume of the bubble ‘B’, we get
⇒(12)(34π(4×10−2)3)=nBRT
This is the ideal gas equation for the bubble ‘B’.
In the question we are asked to find the ratio of number of moles of air in bubbles B and A. for that let us divide the ideal gas equation for the bubble ‘A’ by the ideal gas equation for the bubble ‘B’, i.e.
PBVBPAVA=nBRTnART
⇒(12)(34π(4×10−2)3)(16)(34π×(2×10−2)3)=nBRTnART
By eliminating the common terms in the equation, we get
⇒(12)(4×10−2)3(16)(2×10−2)3=nBnA
By solving this we get the ratio of nAnB as,
⇒nBnA=61
We need to find nAnB. This is the reciprocal of nBnA. Therefore,
⇒nAnB=6
Hence the ratio of the number of moles of air in bubbles B and A is 6.
Note:
Consider a soap bubble of radius ‘r’ as shown below.
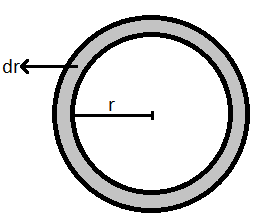
Let ‘T’ be the surface tension of the soap bubble.
Let ‘Pi’ be the pressure inside the bubble and ‘P0’ be the pressure outside the bubble.
We know that due to surface tension the surface molecules of the bubble experience a net force inwards and thus the pressure inside the bubble will be greater than the pressure outside.
Then we get the excess pressure inside the bubble will be,
P=Pi−P0
Due to this excess pressure, the free surface will expand outwards. Let this expansion be ‘dr’
We know that the work done in displacing the excess pressure is stored as the potential energy.
Therefore the small work done,
dW=P×A×dr, where ‘P’ is the excess pressure, ‘A’ is the surface area and ‘dr’ is the displaced surface.
We know that A=4πr2
Therefore,
⇒dw=P×4πr2×dr
Then the increase in the potential energy due to the work done,
dU=TA′, where ‘T’ is the surface tension and ‘A′’ is the increase in the area of the free surface.
We know that A′=2(4π(r+dr)2−4πr2)
Therefore,
⇒dU=T×(2(4π(r+dr)2−4πr2))
⇒dU=T(2(4π(2rdr)))
Since dw=dU, we get
⇒P×4πr2×dr=T(2(4π(2rdr)))
⇒P×4πr2×dr=T16πrdr
⇒P×r=4T
⇒P=r4T
Therefore the pressure inside the soap bubble, P=r4T
