Question
Question: Two small balls having equal positive charges Q on each are suspended by two insulating strings of e...
Two small balls having equal positive charges Q on each are suspended by two insulating strings of equal length L from a hook fixed to a stand. The whole set up is taken in a satellite into space where there is no gravity (state of weightlessness). The angle between the two strings is… and the tension in each string is….. Newton.
Solution
We know that the like charges repel each other and unlike charges attract each other. When charges are at rest, the force between them is given by Coulomb's law.
Formula used:
According to coulomb’s law, the magnitude of electrostatic force between the charges is given by,
F∝r2q1q2
F=kr2q1q2 …………. (1)
Where k is a constant of proportionality called electrostatic force constant. F is the force, q1q2 charges, d is the distance
We have k=4πε01
Substituting the value of k is equation 1 we get,
From equation (1), F=4πε01r2q1q2 ……………(2)
Complete step by step answer:
Let us consider two positive charges +Q is suspended by a two insulating string and charges are separated.
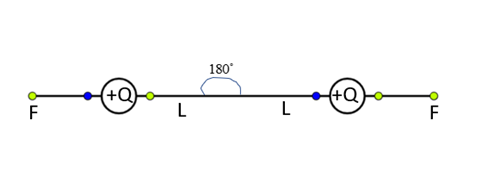
By a distance L from the fixed-point O. Now we can observe the force of repulsion between the charges and they are trying to move away from each other by applying force F.
Now the whole set up is taken in a satellite into space where there is no gravity (state of weightlessness), then mg=0
In the absence of gravitational force, they stand horizontal and opposite to each other at an angle 180∘.
Therefore, the angle between the two strings is 180∘.
The Force of repulsion between the positive charges is F, which equal to the tension in the string
Thus electrostatic force=tension in the string
Since there is no gravity.
We are aware of the coulomb’s law; it states that the electrostatic force between two stationary point charges is directly proportional to the product of their magnitudes and inversely proportional to the square of the distance between their centers.
Electrostatic force, F=4πε01r2q1q2
Then, using the equation, where, q1=Q, q2=Q and r=2L= distance between two charges.
We get,
F=T
⇒4πε01(2L)2Q2
∴ Tension in each string T=4πε01(4L2)Q2
Note:
Coulomb’s force will be repulsive or attractive depending upon whether the charges are like or unlike charges. Further, the force always acts along the line joining the centers of the two charges.
The electrostatic force is strongest compare to gravitational force. The weight of a body is measured by the reaction of the surface which supports the body. The absence of a reaction on a body produces weightlessness.
