Question
Question: Two reactions, A → Products and B → Products, have rate constants $K_a$ and $K_b$ at temperature T a...
Two reactions, A → Products and B → Products, have rate constants Ka and Kb at temperature T and activation energies Ea and Eb respectively. If Ka>Kb and Ea<Eb and assuming that A for both the reactions is same then :
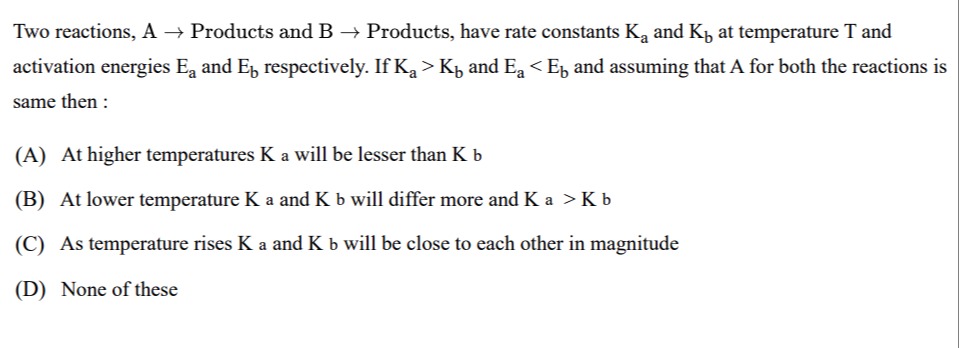
A
At higher temperatures K a will be lesser than K b
B
At lower temperature K a and K b will differ more and K a > K b
C
As temperature rises K a and K b will be close to each other in magnitude
D
None of these
Answer
Options (B) and (C).
Explanation
Solution
Using the Arrhenius equation:
k=Aexp(−E/RT)
If the pre‐exponential factors (A) are the same for both reactions, then:
ka/kb=exp[(Eb–Ea)/(RT)]>1 since Ea<Eb
Thus, at any temperature ka>kb. However, note that:
- At lower temperatures, the exponential term becomes very significant, so the difference between ka and kb is large.
- As temperature increases, the factor (E/RT) decreases, making the difference between ka and kb smaller (their magnitudes get closer).
