Question
Question: Two moles of helium gas undergo a cyclic process as shown in figure. Assuming the gas to be ideal. T...
Two moles of helium gas undergo a cyclic process as shown in figure. Assuming the gas to be ideal. The net work done by the gas is
A. 200RIn2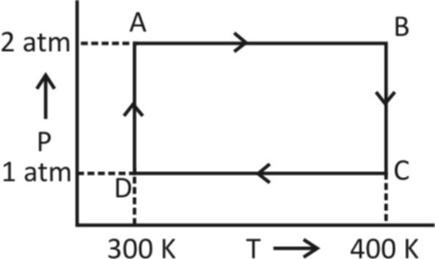
B. 100RIn2
C. 300RIn2
D. 400RIn2
Solution
Concept of work done in case of isothermal process and isobaric process. As the processes AB and CD occur at constant pressure so it is barbaric while processes BC and DA occur at constant temperature so isothermal
Formula used:
1. Work done in an isobaric process
W=PΔV=nRΔT
2. Work done in an isothermal process
=nRTℓnP2P1
Complete step by step answer:
1. Isobaric Process – A process which occurs under constant pressure is called isobaric process.
2. Isothermal Process- A process which occurs for the same temperature is called isotherm process.
3. Isochoric Process- A process for which volume remains constant is called isochoric process.
4. Adiabatic Process- A process which involves no exchange of heat is called adiabatic from now, from the given figure, it is clear that both AB and CD processes involve no change in pressure. So, AB and CD represent isobaric processes.
Work done = PΔV=nRΔT
Where is pressure, R is gas constant, n is number of moles, Δ→change, V is volume, T is temperature.
Now, work done for AB is given by
WAB=nR(T2−T1)
⟹WAB=nR(400−300)[asT1=300K,T2=400K]
WAB=nR100.........(1)
Work done for CD is given by
WCD=nRΔT
⟹WCD=nR(T1−T2)
Putting the values of temperatures
WCD=nR(300−400)
⟹WCD=nR100.......(2)
Now, the process BC and DA occur at a constant temperature. So BC and DA represent isothermal process and the work done by an isothermal process is given by
W=nRTℓnP2P1
Where P1 is the initial pressure
P2 is the final pressure
Now, work done for isothermal process BC is given by
WBC=nRTℓnP1P2
⟹WBC=nRTℓn21(ashereP1=2atm,P2=1atm)
⟹WBC=400nRℓn21(ashereT=400K)
⟹WBC=400nR(ℓn1−ℓn2)
On solving this we get
WBC=400nR(0−ℓn2)
⟹WBC=400nRℓn2..........(3)
Now, work done for isothermal process DA is
WDA=nRTℓn12
⟹WDA=300nRℓn2........(4)
Total work done =WAB+WBC+WCD+WDA
=100nR−400nRℓn2−100nR+300nRℓn2
=−100nRℓn2
Here number of moles, n=2
So, Net work done =−100×2Rℓn2
=−200Rℓn2
So, the correct answer is “Option A”.
Note:
Remember ℓn(ba)=ℓna−ℓnb and ℓn(ab)=ℓna+ℓnb
So, ℓn(21)=ℓn1−ℓn2that is basic formulas of logarithms are used.
