Question
Question: Two moles of Helium gas are taken over the cycle \(ABCDA\) as shown in the P-T diagram. The net work...
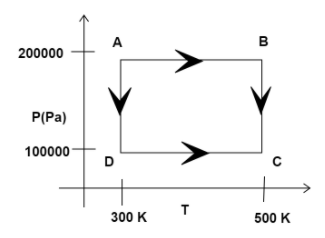
Two moles of Helium gas are taken over the cycle ABCDA as shown in the P-T diagram. The net work done on the gas in the cycle ABCDA IS:
A.0
B.276R
C.1076R
D.1094R
Solution
To find the work done in the process, we need to find the work done at respective stages that is work done inAB, BC, CD,DA. The summation of the work done at this stage will give us the total work done by the gas.
Formula used:
W=nRΔT
W= Work done
n= Number of moles
R= Universal Gas constant
ΔT= Change in temperature.
W=nRTln(P2P1)
W= Work done
n= Number of moles
R= Universal Gas constant
ΔT= Change in temperature
P1= Pressure at point 1
P2= Pressure at point 2
Complete step by step answer:
We know, this is a cyclic process as in this the starting and the ending point are same.
Now, let us consider stageAB:
As we can see from the diagram, the pressure remains constant but the temperature changes.
Therefore, the work done in ABis
WAB=nRΔT
Now, putting the values we get:
WAB=2×R×(500−300)=400R
In case of BC, the process takes place at constant temperature, but changing pressure.
Thus, we know, the formula for work done in BC is written as:
WBC=nRTln(PCPB)
Now, putting the values, we get:
WBC=2×R×500×ln(2)=690R
Again, in case ofCA, as we can see from the diagram, the pressure remains constant but the temperature changes.
Thus, work done byCA, is given by:
WCA=2×R×(300−500)=−400R
Now, again:
In case of DA, the work done is:
WDA=n×R×T×ln(PAPD)
On putting the values, we get:
WDA=2×R×500×ln(0.5)=−414R
Therefore, total work done by the system is:
W=WAB+WBC+WCD+WDA
Now, putting the values as obtained above:
W=400R+690R−400R−414R
Therefore, the obtain work done is 276R
Thus, option (B) is correct.
Note:
Since in stage AD and CB as we can see from the diagram, the pressure remains constant but the temperature changes, they are known as isochoric process and in case of AB and DA , the pressure remains constant but the temperature changes, the process is known as isobaric process.
