Question
Question: Two moles of helium gas are taken over the cycle \(ABCDA\), as shown below in the \(P - T\) diagram....
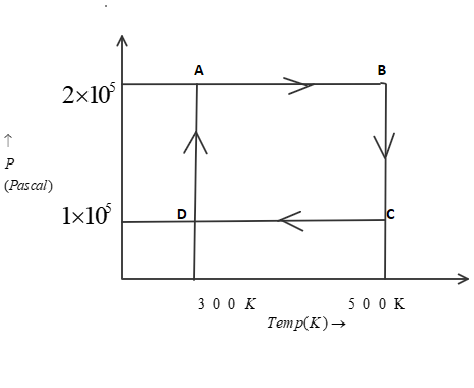
Two moles of helium gas are taken over the cycle ABCDA, as shown below in the P−T diagram. (Assume the gas to be ideal and R is gas constant).
Now, match List I with List II and select the options given below:
| List I | List II |
|---|---|
| (P) | Magnitude of work done on the gas in taking from A→B |
| (Q) | Magnitude of work done on the gas in taking from B→C |
| (R) | Magnitude of work done on the gas in taking from D→A |
| (S) | Magnitude of the net heat absorbed/evolved in the cycle ABCDA |
A.) P−1,Q−2,R−3,S−4
B.) P−3,Q−2,R−4,S−1
C.) P−3,Q−1,R−4,S−2
D.) P−4,Q−3,R−1,S−2
Solution
When temperature remains constant and other parameters change then this process is called an isothermal process and when pressure remains constant then this process is known as an isobaric process.
Complete step by step answer:
There are some thermodynamic processes that are used in this question. These are:
An isobaric process is a thermodynamic process in which pressure remains constant and volume is expanded or contracted. The work done in an isobaric process can be given by the formula as follows:
Wisobaric=−nR(T2−T1)
Where, n= number of moles
R= Real gas constant
T2= final temperature
T1= Initial temperature
An isothermal process is a thermodynamic process in which the temperature of a system remains constant and pressure may increase or decreases. The work done in an isothermal process can be given as:
Wisothermal=−2.303nRTlog(P2P1)
Where, P1= Initial pressure
P2= Final pressure
n= number of moles
R= Real gas constant
Now, In part P.) to find work done from A→Bwe know that pressure is constant as we move from point A to point B. Therefore, it is an isobaric process. So, we can use this equation:
Wisobaric=−nR(T2−T1)
As given in question, that number of moles(n) is 2, also the initial temperature(T1) is 300K and final temperature (T2) is 500K. Now, by putting all these values in above equation, we get:
WA→B=−2R(500−300) =−400R
Now, In part Q.) to find work done from B→C we know that temperature is constant as we move from point B to point C. Therefore, it is an isothermal process. So, we can use this equation:
Wisothermal=−2.303nRTlog(P2P1)
As given in question, that number of moles(n) is 2, also the initial pressure(P1) is 2×105P and final pressure (P2) is 1×105P. Now, by putting all these values in above equation, we get:
WB→C=−2.303×2R×500×log(1×1052×105)
=−693R
Now, In part R.) to find work done from D→A we know that temperature is constant as we move from point D to point A. Therefore, it is an isothermal process. So, we can use this equation:
Wisothermal=−2.303nRTlog(P2P1)
As given in question, that number of moles(n) is 2, also the initial pressure(P1) is 1×105P and final pressure (P2) is 2×105P. Now, by putting all these values in above equation, we get:
WB→C=−2.303×2R×300×log(2×1051×105)
=+416R
Now to find work done from C→Dwe know that pressure is constant as we move from point C to point D. Therefore, it is an isobaric process. So, we can use this equation:
Wisobaric=−nR(T2−T1)
As given in question, that number of moles(n) is 2, also the initial temperature(T1) is 500K and final temperature (T2) is 300K. Now, by putting all these values in above equation, we get:
WC→D=−2R(300−500) =+400R
Now, to find net heat evolved or absorbed, we will add all work done in the cycle ABCDA.
Therefore, Net heat =WA→B+WB→C+WC→D+WD→A
=(−400)+(−693)+(+400)+(+416) =−277R
Hence, Option C.) is the correct answer.
Note: Remember that in this question we are asked to find the magnitude of the work done or heat evolved or released. Therefore, we will take all work done and heat evolved or released as positive to get the required answer.
