Question
Question: Two moles of an ideal monoatomic gas undergoes a cyclic process represented by the following P-U dia...
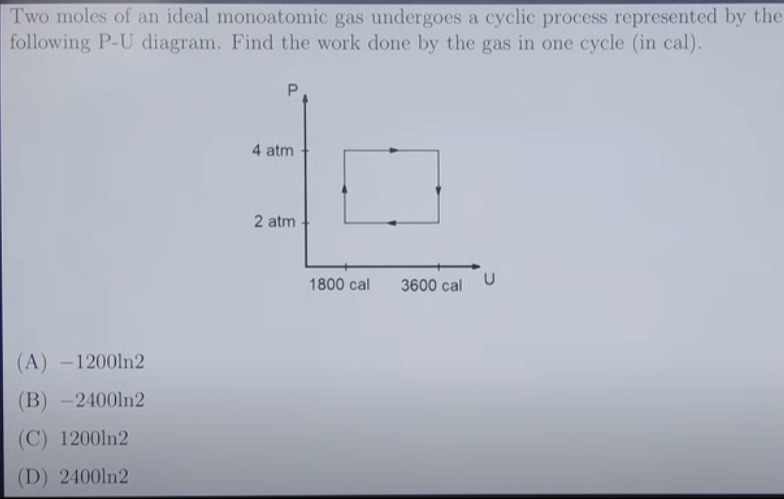
Two moles of an ideal monoatomic gas undergoes a cyclic process represented by the following P-U diagram. Find the work done by the gas in one cycle (in cal).
-1200ln2
-2400ln2
1200ln2
2400ln2
-1200ln2
Solution
The problem asks for the work done by two moles of an ideal monoatomic gas undergoing a cyclic process shown on a P-U diagram.
For an ideal monoatomic gas, the internal energy U is related to pressure P and volume V by the equation: U=23nRT
Also, from the ideal gas law, PV=nRT. Substituting nRT into the internal energy equation, we get: U=23PV
From this, we can express volume V as: V=3P2U
Let's identify the coordinates of the four corners of the cycle from the P-U diagram: Point A: (U1,P1)=(1800 cal,2 atm) Point B: (U2,P1)=(3600 cal,2 atm) Point C: (U2,P2)=(3600 cal,4 atm) Point D: (U1,P2)=(1800 cal,4 atm)
Now, let's calculate the volume at each point using V=3P2U: VA=3×2 atm2×1800 cal=63600atmcal=600atmcal VB=3×2 atm2×3600 cal=67200atmcal=1200atmcal VC=3×4 atm2×3600 cal=127200atmcal=600atmcal VD=3×4 atm2×1800 cal=123600atmcal=300atmcal
The cyclic process consists of four steps as indicated by the arrows: A → B → C → D → A.
1. Process A to B (Isobaric expansion): Pressure P is constant at P1=2 atm. Work done WAB=P1(VB−VA) WAB=2 atm×(1200−600)atmcal=2×600 cal=1200 cal
2. Process B to C (Isothermal compression): Internal energy U is constant at U2=3600 cal. For an ideal gas, constant U implies constant temperature T (isothermal process). Work done WBC=nRTln(VBVC) From U=23nRT, we have nRT=32U. So, nRT=32×3600 cal=2400 cal. WBC=2400 cal×ln(1200600)=2400ln(21)=−2400ln(2) cal
3. Process C to D (Isobaric compression): Pressure P is constant at P2=4 atm. Work done WCD=P2(VD−VC) WCD=4 atm×(300−600)atmcal=4×(−300) cal=−1200 cal
4. Process D to A (Isothermal expansion): Internal energy U is constant at U1=1800 cal. This is an isothermal process. Work done WDA=nRTln(VDVA) From U=23nRT, we have nRT=32U. So, nRT=32×1800 cal=1200 cal. WDA=1200 cal×ln(300600)=1200ln(2) cal
Total work done in one cycle: The total work done by the gas in one cycle is the sum of the work done in each process: Wcycle=WAB+WBC+WCD+WDA Wcycle=1200 cal+(−2400ln(2) cal)+(−1200 cal)+(1200ln(2) cal) Wcycle=(1200−1200)+(−2400ln(2)+1200ln(2)) Wcycle=0−1200ln(2) cal Wcycle=−1200ln(2) cal
The work done by the gas in one cycle is −1200ln(2) cal.
