Question
Question: Two longest wavelengths (in nanometre) in the Lyman series of the hydrogen spectrum, are...
Two longest wavelengths (in nanometre) in the Lyman series of the hydrogen spectrum, are
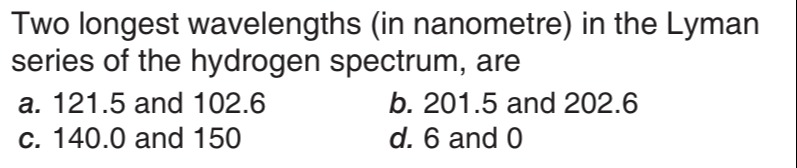
121.5 and 102.6
201.5 and 202.6
140.0 and 150
6 and 0
121.5 and 102.6
Solution
The wavelengths in the hydrogen spectrum are given by the Rydberg formula:
λ1=R(nlower21−nhigher21)
where R is the Rydberg constant, nlower is the principal quantum number of the lower energy level, and nhigher is the principal quantum number of the higher energy level (nhigher>nlower).
For the Lyman series, the electron transitions to the ground state, so nlower=1. The formula becomes:
λ1=R(121−nhigher21)=R(1−nhigher21)
where nhigher=2,3,4,….
The wavelength λ is longest when the energy difference is smallest. The smallest energy difference in the Lyman series corresponds to the transition from the lowest possible higher energy level, which is nhigher=2.
The longest wavelength (λ1) corresponds to the transition from nhigher=2 to nlower=1:
λ11=R(1−221)=R(1−41)=R(43)
λ1=3R4
The second longest wavelength (λ2) corresponds to the transition from the next lowest higher energy level, which is nhigher=3 to nlower=1:
λ21=R(1−321)=R(1−91)=R(98)
λ2=8R9
To calculate the wavelengths in nanometers, we use the value of the Rydberg constant R≈1.09677×107m−1.
λ1=3×1.09677×107m−14≈3.29031×1074m≈1.21567×10−7m
Converting to nanometers: 1.21567×10−7m×1m109nm≈121.567nm.
λ2=8×1.09677×107m−19≈8.77416×1079m≈1.02570×10−7m
Converting to nanometers: 1.02570×10−7m×1m109nm≈102.570nm.
Using a slightly more precise value of R from the similar question, R=109678cm−1=1.09678×107m−1:
λ1=3×1.09678×107m−14≈1.21565×10−7m=121.565nm.
λ2=8×1.09678×107m−19≈1.02569×10−7m=102.569nm.
Rounding these values to one decimal place gives 121.6 nm and 102.6 nm. Comparing with the options, option (a) has values 121.5 and 102.6. These are the closest values.
The two longest wavelengths in the Lyman series are approximately 121.6 nm (from n=2 to n=1) and 102.6 nm (from n=3 to n=1).
