Question
Question: Two identical masses of 5kg each wheel falls from a height of 10m. The wheel disturbs a mass of 2kg ...
Two identical masses of 5kg each wheel falls from a height of 10m. The wheel disturbs a mass of 2kg water, the rise in the temperature of the water will be:
1. 2.6∘C
2. 1.2∘C
3. 0.32∘C
4. 0.12∘C
Solution
According to the law of conservation of energy. Energy can neither be created nor destroyed it can be just transferred from one form to another. When there is the transfer of energy some energy is dissipated in the form of heat and simultaneously the change in the temperature of the system can be observed.
Formula used:
The potential energy of a body at a certain height
EP=mwlsgh
The energy of the water system,
E=mwtrSΔT
Complete answer:
The amount of heat required to raise the temperature of 1 gram substrate by 1∘C is known as the specific heat of the material. Water has a high specific heat which is also known as the heat capacity of the substrate.
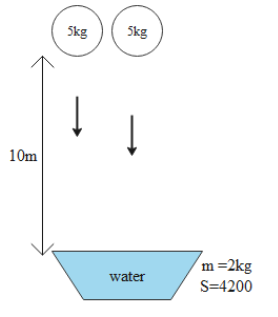
Water has a high heat capacity. The heat capacity of water is around 4200 J/Kg – C
As per the law of conservation of the energy, the rise in the temperature will be such that it will compensate for the change in energy equivalent to the potential energy of the wheels when situated at a height 10m above the reference line.
Mathematically,
mwlsgh=mwtrSΔT
Where,
mwls is the total mass of the wheels
g is the acceleration due to gravity
h is the height at which wheels were situated
mwtr is the mass of the water disturbed
S is the specific heat of the water
ΔT is the change in temperature of the water.
By rearranging the change in the temperature can be given as,
ΔT=mwtrSmwlsgh⇒ΔT=2(4200)1000ΔT=0.12∘C
So the temperature change is 0.12∘C.
The correct option for the question is Option 4.
Note:
The specific heat of the water is always the same. The change in temperature is a result of energy loss. When a particular experiment or task is done some amount is dissipated as heat loss which and gradually the temperature of the body increases according to the same.
