Question
Question: Two identical glass bulbs are interconnected by a thin glass tube at \(0{}^\circ C\). A gas is fille...
Two identical glass bulbs are interconnected by a thin glass tube at 0∘C. A gas is filled at N.T.P. in these bulbs is placed in ice and another bulb is placed in a hot bath, then the pressure of the gas becomes 1.5 times. The temperature of the hot bath will be
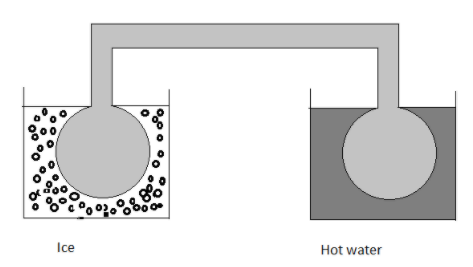
Solution
There is an exchange between the system and surrounding to balance the energy (heat) within them. The energy flows from higher concentration to lower concentration. If the pressure or temperature around an object increases or not the number of molecules (moles) always remains the same (constant).
Complete answer:
According to the ideal gas equation which gives a relation between the pressure, volume, no of moles, gas constant, and temperature. At N.T.P. the relation would be,
P0V0=nRT0
The no of moles at NTP will be,
n=RT0P0V0...(1)
So when we look at the situation mentioned in the question two bulbs are kept in the surrounding of different temperatures.
So, the no of moles of the gas-filled in the bulb in an ice bucket can be given as,
n1=RT01.5P0V0...(2)
And, the no of moles of the gas-filled in the bulb in hot water bucket can be given as,
n2=RT1.5P0V0....(3)
The no of molecules in the gas which is filled in both the bulbs always remains the same.
n1+n2=n+n
So from equation (1), (2), and (3), we can write the above equation as,
RT01.5P0V0+RT1.5P0V0=RT0P0V0+RT0P0V0⇒T01.5+T1.5=T02∴T=3(T0)
At N.T.P the value of temperature is considered as 273K. so the temperature of the hot water basket will be,
T=3(273)∴T=891K=546∘C
Thus, the required answer to the question is 891K or 546∘C
Note:
N.T.P. stands for Normal temperature and pressure, this can be also termed as STP (standard temperature and pressure). These abbreviations are used for the condition when the measurement of the characteristics or gas are taken at a standard value of temperature (273K) and pressure (1atm). These values always remain constant for all the standard calculations and all the gases.
