Question
Question: Two identical breakers with negligible thermal expansion are filled with water of the same level at ...
Two identical breakers with negligible thermal expansion are filled with water of the same level at {\text{4^\circ C}} if A is heated while the other B is cooled, then:
(A) Water level in A must rise
(B) Water level in B must rise
(C) Water level in A must fall
(D) Water level in B must fall
Solution
Hint : For the solution, we use anomalous properties of water to find the level of breaker A and B, which are filled with water of the same level at {\text{4^\circ C}} in this one, is heated and cooled. By using graphs, we check the density of water according to temperature increment and decrement. If density decreases, then volume increases and vice versa.
Complete Step By Step Answer:
A common observation seen within the behavior of the substances is that they expand when heated because the density decreases, and the other way around takes place when the fabric is cooled. This is how substances generally react to heat. Now look at how water behaves when it is heated.
The general tendency of cold water remains unchanged until the density of water gradually increases as you cool it. When you reach its density reaches a maximum. What water does next will astound you. When you calm further to form some ice, i.e. water expands with an extra drop by temperature, meaning the density of water decreases.
The effect of this expansion of water is that the coldest water is usually present on the surface. Since water is the heaviest, this water settles on the rock bottom of the water body and therefore the lightest i.e. the coldest layer accumulates on the highest layer. So, within the winter, the highest of the water is usually the primary to freeze over. Since ice and water both are bad conductors of warmth, this top layer of ice insulates the remainder of the water body from the cold of the winter, thereby protecting all the life in the water body. Now you'll truly comprehend how essential the anomalous properties of water are for all times.
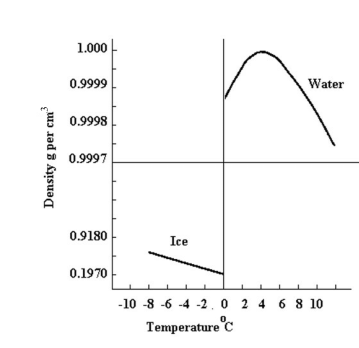
Water has maximum density at {\text{4^\circ C}} a common observation seen in the behavior of water is that they expand when heated i.e. volume increases. Then the water level in A must rise due to heating. Hence option A is correct.
According to anomalous properties of water, when water is cooled below {\text{4^\circ C}} (as shown in above graph) then the density of water decreases i.e. volume increases. Hence the water level in B must increase. Hence option B is correct.
When water is heated above {\text{4^\circ C}} then density of water decreases i.e. volume increases. Hence water level in A must increase. Then option C is incorrect.
When water is cooled below {\text{4^\circ C}} then density of water will decrease i.e. volume increase. Hence water level in B must increase. Then option D is incorrect.
Option A, B is correct.
Note :
Generally with decrease in temperature the density for any fluid decreases but water shows us an exception. While doing such problems, if we have to do some kind of numerical calculations, if we have to use temperature, then we always take in Kelvin. Also, if the process is isothermal then during expansion or compression the temperature does not change. Density is the ratio of mass and volume and mass does not change at all. If the volume of the breaker increases then the level of the breaker must rise.
