Question
Question: Two identical beakers A and B contain equal volumes of two different liquids at \(60^{\circ}\) each ...
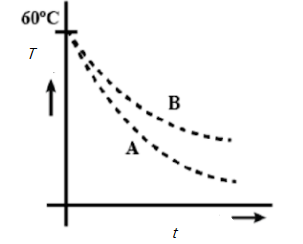
Two identical beakers A and B contain equal volumes of two different liquids at 60∘ each and left to cool down. Liquid in A has density 8×102kgm−3 and specific heat 2000Jkg−1K−1 while liquid B has density 103kgm−3 and specific heat 4000Jkg−1K−1. Which of the following best describes their temperature versus time graph schematically? (Assume the emissivity of both the beakers to be the same).
A.
B.
C.
D.
Solution
Newton’s laws of cooling states that the rate of heat loss of a body is directly proportional to the difference in the temperature between the body and its surroundings. We can solve the problem by using Newton’s law of cooling and the first law of thermodynamics.
Formula used:
dtdU=CdtdT
dtdT=(ρ×v)×cQ
Complete step by step answer:
Newton’s laws of cooling states that the rate of heat loss of a body is directly proportional to the difference in the temperature between the body and its surroundings.
He also noted that, with some mathematical manipulation of the law, he found that the rate of temperature change of a body is proportional to the difference in temperatures between the body and its surroundings . This was done to override the confusion in Newton's time between the concepts of heat and temperature.
He gave that C=dTdU, where C is the heat capacitance, U is the internal energy of the system, and T is the temperature.
Differentiating U wrt time t, we get
dtdU=CdtdT
We know that, C=mc and m=ρ×v , where m is mass of the substance, ρ is density and v is volume of the substance and c is specific heat of the substance.
Also, from the first law of thermodynamics dvUt=−Q i.e. loss of heat energy to the surroundings. Since both A & B beakers are left to cool in the same conditions, Q is the same for both the beakers.
Then Q=(ρ×v)×cdtdT
dtdT=(ρ×v)×cQ
Since volume and loss of heat is same for both the beakers
dtdT=ρ×ck;k=vQ
For BA=dtdT2dtdT1=ρ1c1ρ2c2 givenρ1=8×102kgm−3;c1=2000Jkg−1K−1ρ2=103kgm−3;c2=4000Jkg−1K−1
BA=dtdT2dtdT1=8×102×2000103×4000=16×1054×106
Means dtdT1>dtdT2
Hence A is the answer.
Note:
Be careful in interpreting the results graphically. Be careful with the symbols and notations used. Try to remember the principle and the formulas used. Remember that the law is converted into two forms.
