Question
Question: Two geometrical isomers are given by the following compound A. Ethylidene bromide B. Acetylene t...
Two geometrical isomers are given by the following compound
A. Ethylidene bromide
B. Acetylene tetrachloride
C. Acetylene tetrabromide
D. Acetylene dibromide
Solution
The chemical which has the same chemical formula and exists in different forms is called isomers. The compounds or chemicals which have a double bond in its structure generally show geometrical isomerism.
Complete step by step answer:
- In the question it is asked which molecules among the given options will show two geometrical isomers.
- To find it we have to draw each and every structure of the compound given in the options.
- Coming to option A, Ethylidiene bromide.
- The structure of ethylidene bromide is as follows:
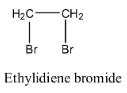
- In the above structure we can clearly see that there is no double bond present in it.
- Therefore option A does not show that geometrical isomerism.
- Coming to option B, acetylene tetrachloride.
- The structure of acetylene tetrachloride is as follows.
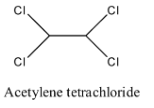
- In the structure of option B, there is no double bond.
- Therefore acetylene tetrachloride is not going to exhibit geometrical isomerism.
- Coming to option C, Acetylene tetrabromide.
- The structure of Acetylene tetrabromide is as follows:
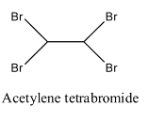
- In the structure of option C, there is no double bond.
- Therefore acetylene tetrabromide is not going to exhibit geometrical isomerism.
- Coming to option D, Acetylene dibromide.
- The structure of Acetylene dibromide is as follows.
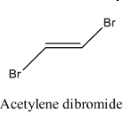
- In the structure of the acetylene dibromide there is a presence of double bond.
- Therefore acetylene dibromide is going to show geometrical isomerism.
- The geometrical isomerism of the compound acetylene dibromide is as follows.
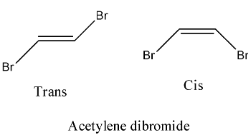
Therefore option D is correct.
Note: The compound which exhibits geometrical isomerism shows two types of isomers. The two types of geometrical isomers are cis and trans. Trans form of a compound is more stable then the cis form because of the less steric hindrance.
