Question
Question: Two different compounds have the formula \[{\text{Xe}}{{\text{F}}_{\text{2}}}{\text{C}}{{\text{l}}_{...
Two different compounds have the formula XeF2Cl2. How do you write the Lewis structures for these two compounds, and describe how measurement of dipole moments might be used to distinguish between them?
Solution
A Lewis Structure is a simplified representation of a molecule's valence shell electrons. It's used to demonstrate how electrons in a molecule are organised around individual atoms. Dipole moments are caused by variations in electronegativity and may occur between two ions in an ionic bond or between atoms in a covalent bond.
Complete answer:
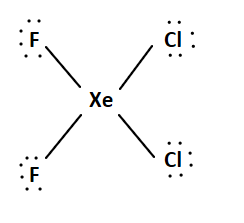
There are 32 valence electrons in the trial structure.
1 Xe + 2 F + 2 Cl = 8 + 14 + 14 = 36 valence electrons
We add two-line pairs to the central atom since we have four extra electrons.
The Lewis structure is:
This system is an AX4E2system. It has an octahedral electron geometry. The F and Cl atoms will occupy the equatorial positions, while the bulky lone pairs will occupy the top and bottom axial positions. The molecule has a square planar geometry. We can arrange the atoms in two different ways.
The F - Xe - F and Cl - Xe - Cl bond angles are 180 degrees in the trans isomer. The Xe - Cl bond dipoles cancel the Xe - F bond dipoles, and the Xe - F bond dipoles cancel the Xe - Cl bond dipoles. The trans isomer is nonpolar since there is no net dipole.
The Xe - F and Xe - Clbond dipoles do not cancel in the cis-isomer. The molecule is polar in the cis isomer, which has a net dipole moment.
Note:
Cis isomers are molecules that have the same atom connectivity. They have side groups that are identical on the same side of a double bond. Molecules with identical side groups on opposite sides of a double bond are called trans isomers.
