Question
Chemistry Question on Ideal gas equation
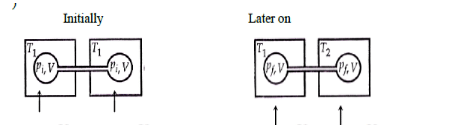
Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T2. The final pressure pf is :
A
pi(T1+T2T1T2)
B
2pi(T1+T2T1)
C
2pi(T1+T2T2)
D
2pi(T1+T2T1T2)
Answer
2pi(T1+T2T2)
Explanation
Solution