Question
Question: Two cells of 1.25V and 0.75V are connected in parallel. The effective voltage will be?...
Two cells of 1.25V and 0.75V are connected in parallel. The effective voltage will be?
Solution
We have to use the Kirchhoff’s rule for finding the effective voltage when two cells are connected in parallel. In parallel grouping of cells, all anodes are connected at one point and all cathodes are connected together at other points. If n identical cells are connected in parallel then effective voltage of parallel combination is equal to the e.m.f. of one cell.
Complete step by step answer:
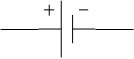
This figure represents a cell. Cell always contains two terminals one is a positive terminal and one is a negative terminal. Cell is always the source from where the voltage is produced. A cell converts chemical energy into electric energy. Combination of cells gives a battery. When one negative terminal of a cell is connected to the next positive terminal of a cell then it is called a series combination of cells while when all positive terminals and negative terminals of many cells are connected together then such combination is called parallel combination.
Cells always provide a Direct Current. Current flow in circuits only when charge carriers travel around the circuit. Current is defined as the rate at which this charge passes any point in the circuit.
There is basically two Kirchhoff’s Law: -
Junction rule: - The sum of incoming current towards the junction is always equal to sum of outgoing current away from junction.
Loop Rule: - This rule is applicable only for Closed loop, according to this law algebraic sum of all the potential difference of all the components used in a closed loop is always equal to zero.
Here in this question we have given two cells. Each of different voltages are connected in parallel combination.
Let us assume the voltage of the 1st cell as V1and the 2nd voltage asV2.
So according of the question values of both the cells can be written as:-
