Question
Question: Two aqueous solutions are mixed in a chemical reaction. The substances in this beaker represent the ...
Two aqueous solutions are mixed in a chemical reaction. The substances in this beaker represent the products of this chemical reaction. Which substance(s) in the particulate-level diagram is a spectator ion?
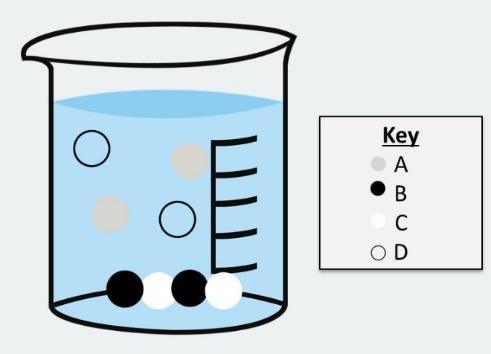
(A) A and D
(B) B and C
(C) D
(D) B
Solution
Spectator ions can be either cations (positively-charged ions) or anions (negatively-charged ions). On both sides of a chemical equation, the ion is unchanged and does not affect equilibrium. Spectator ions detected in the original equation are ignored when writing a net ionic equation. The total ionic reaction, therefore, is distinct from the net chemical reaction.
Complete Step-by-Step solution :
By looking at the figure provided to us, we can see that there are four types of substance present in the beaker. They are A, B, C, and D. Also, we can observe that substances A and D are in the middle of the beaker or we can say they are very well mixed up in the liquid while substances B and C are settled at the bottom of the beaker.
We can observe that the substance A and D are a part of the chemical reaction. We can say this because both these substances A and D are found to be in the middle of the beaker. That is, they are taking part in the chemical reaction and they are not separate from the chemical process.
While looking at substances B and C, we can see that both these substances are settled at the bottom of the beaker during the mixture of the two aqueous solutions. We can say that substances B and C are acting as precipitate and it is also taking part in the chemical reaction.
Now, if we go through the basic definition of the spectator ion, we know that spectator ions have to be in the same phase of the reaction mixture. That is, as per our question, they have to be in a liquid phase.
So, we can conclude that substance A and substance D are in the same phase as the reaction mixtures, that is, the liquid phase. Hence, they are the spectator ions.
While substance B and C are precipitates in the beaker and they are not in the same phase of the reaction mixture.
Hence, the correct option is (A).
Note:
The chemical species that undergo a chemical change are indicated by the net ionic equation. On both sides of the equation, the ions that appear remain constant and are therefore considered spectator ions.
