Question
Question: Two acids have been derived from \({{H}_{2}}{{O}_{2}}\) by replacing H by (\(S{{O}_{2}}OH\)) group. ...
Two acids have been derived from H2O2 by replacing H by (SO2OH) group. Both the acids have one peroxy linkage
H−O−O−H,H−O−O−SO2−OH (H2SO5)
H−O−O−H,HO−SO2−O−O−SO2−OH(H2S2O8)
Based on the above study, answer the following questions;
Above those 2 compounds, which is called Marshall’s acid and which is called Caro’s acid
Solution
The peroxy acids: H2SO5 is called as peroxymonosulfuric acid and H2S2O8 is called as the peroxydisulfuric acid.
The names Marshall’s acid and Caro’s acid came after the names of their inventors.
Complete answer:
So this is a fact based question in which two acids are given and their bonds connectivity is also given. And we have to say that among the two acids that is derived from hydrogen peroxide, which one is called the Caro's acid and which one is called the Marshall’s acid.
So here we know that H2SO5 is also called as the peroxymonosulfuric acid, there is only one SO2OH group in this molecule hence the term mono is given in the nomenclature of the peroxy acid.
It was first described by Heinrich Caro and hence the peroxymonosulfuric acid (H2SO5) was called the Caro’s acid.
The structure of Caro’s acid is:
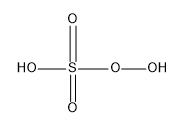
The S which is the central atom in the molecule adopts the tetrahedral geometry; the S atom here has an oxidation number of +6.
Now let’s move to the next peroxy acid H2S2O8, which is called as peroxydisulfuric acid.
The molecule is called so since there are two SO2OH groups present in the structure of this molecule.
This acid is called Marshall's acid as it was first described by Professor Hugh Marshall, as a mark of appreciation and respect for his invention, the peroxydisulfuric acid is generally called as Marshall’s acid.
Here also the sulphur atom forms six bonds and the compound H2S2O8 is commonly called as persulphates.
The structure of H2S2O8 is as follows
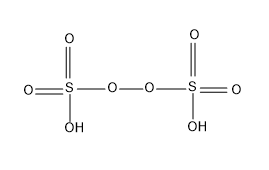
Note:
There is very high chance of getting confused between Caro’s acid (H2SO5) and Marshall’s acid (H2S2O8) since both of them are peroxy acids and only differ by one unit of SO2OH group.
Both the acids are very potent oxidizing agents.
