Question
Question: TThe degree of unsaturation of phenetole is: A.3 B.6 C.4 D.5...
TThe degree of unsaturation of phenetole is:
A.3
B.6
C.4
D.5
Solution
Degree of unsaturation is the number of cyclic rings and bonds present in a molecule. Phenetole is also known as Ethyl phenyl ether is an organic compound that is ether. Phenetole exhibits the properties such as volatility, explosive vapors, and the ability to form peroxides similar to the other ethers. It will dissolve in less polar solvents such as ethanol but polar solvents such as water.
Complete step by step answer:
In phenetole or ethyl phenyl ether there is one benzene ring which is attached with a ether group (−R−O−R/)
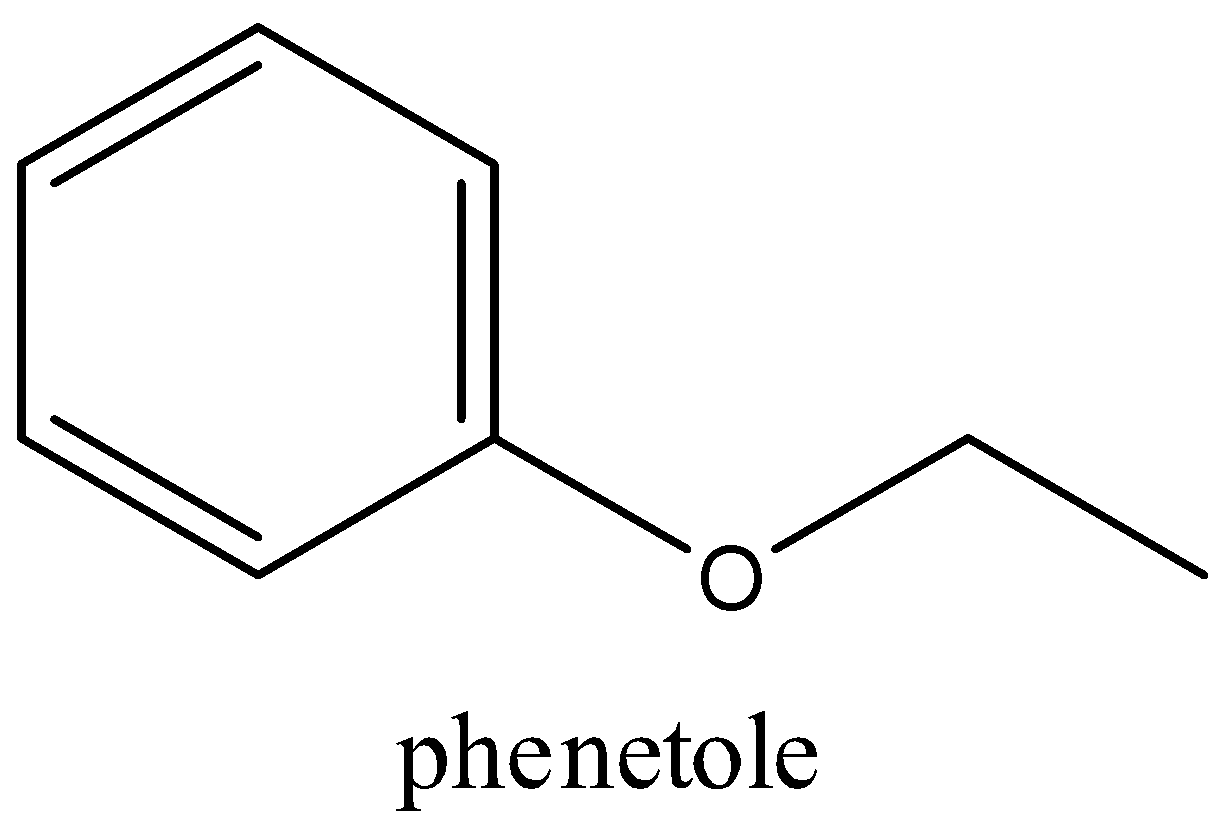
Total number of the cyclic ring in phenetole is 1
Total number of double bonds in phenetole is 3 (the phenyl contains three double bonds)
Degree of unsaturation =1+3
Degree of unsaturation =4
Therefore, the degree of unsaturation of phenetole is 4
So, the correct answer is Option C.
Note:
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is defined as the calculation that helps in determining the total number of rings and π bonds.
The simplified formula for the calculation of the degree of unsaturation
Double Bond Equivalent =(a+1)−2b−c+f
Here,
a is number of carbon atoms in the compound
b is number of hydrogen atoms in the compound
c is number of nitrogen atoms in the compound
f is number of halogen atoms in the compound
In case of hydrocarbons, the DBE or we can say IHD tells us about the number of rings or extra bonds present in a non-saturated structure that is equal to the number of hydrogen pairs required for the formation of a saturated structure. As the addition of two elements to form a ring or addition of extra bond in the structure will reduce the need for two hydrogens.
In case of non-hydrocarbons, the elements in a pair can include any elements from the lithium family or the fluorine family, not necessarily all hydrogens.
