Question
Question: Trigonal bipyramidal geometry is shown by- [A] \(XeO{{F}_{2}}\) [B] \(Xe{{O}_{3}}{{F}_{2}}\) [...
Trigonal bipyramidal geometry is shown by-
[A] XeOF2
[B] XeO3F2
[C] FXeOSO2F
[D] [XeF8]2−
Solution
Hint: To find the geometry of any complex, we need to determine the coordination number of the complex. If we know the coordination number, we can easily find out the hybridization and the geometry of the complex. The correct option is the anionic part of the complex.
Complete answer: Trigonal bipyramidal geometry is seen in complexes which have hybridization ofsp3dand their coordination number is 5.
Let us discuss each option to find out if they are in trigonal bipyramidal geometry or not-
In option [D], we have [XeF8]2−
Number of valence electrons in Xe=8
Contribution of each fluorine atom is 1. Here we have 8 fluorine atoms therefore, for 8 fluorine atoms we will add 8.
We have an overall charge of -2, so we will add 2 to this.
The above mentioned addition will give us the total number of electrons. Dividing it by 2 will give us the coordination number-
∴C.N=(8+8+2)÷2=9
Therefore, the coordination number of the complex is 9.
So, The hybridization=s1p3d5(1+2+5=9)
Shape of [XeF8]2− is square antiprism, which is difficult to draw on a 2d-plane.
In the next option, [C] we have, FXeOSO2F
Number of valence electron on the central atom, Xe= 8
Contribution of each fluorine atom= 1
Contribution of Oxygen and sulphur is 0.
∴C.N=(8+2)÷2=5
According to the calculation, the hybridization is sp3dbut this complex does not exist.
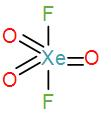
In option [B], we have XeO3F2
Valence electrons in xenon=8
Contribution of each F atoms=2
∴C.N=(8+2)÷2=5
Therefore, the geometry is trigonal bipyramidal. We can draw its structure as-
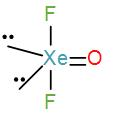
 Lastly, in option [A] we have, XeOF2-
Lastly, in option [A] we have, XeOF2-
Valence electrons in xenon=8
Contribution of each F atoms=2
∴C.N=(8+2)÷2=5
Therefore, the geometry is trigonal bipyramidal. We can draw its structure as-
 Therefore, XeO3F2and XeOF2have trigonal bipyramidal geometry.
Therefore, XeO3F2and XeOF2have trigonal bipyramidal geometry.
Therefore, the correct options are-[A] XeOF2and [B]XeO3F2.
Note: Here, both XeO3F2 and XeOF2 have a trigonal bipyramidal geometry but they have dissimilar structures. XeOF2 is T-shaped and it has 2 lone pairs but in XeO3F2, all the electrons are occupied by oxygen and fluorine. Therefore, shape and geometry should be thought differently.
