Question
Question: Trichloroacetaldehyde, \[CC{l_3}CHO\] reacts with chlorobenzene in presence of sulphuric acid and pr...
Trichloroacetaldehyde, CCl3CHO reacts with chlorobenzene in presence of sulphuric acid and produces:
i.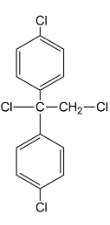
ii.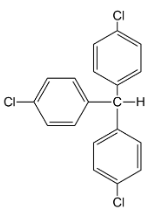
iii.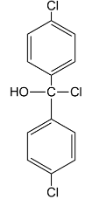
iv.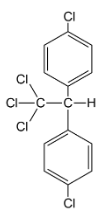
Solution
The reactants given are trichloroacetaldehyde and chlorobenzene, where chlorobenzene attacks the electron deficient carbon centre of the carbonyl group of trichloroacetaldehyde. Sulphuric acid is a dehydrating agent and helps in removal of water molecules. Hence find the product formed.
Complete step-by-step answer: The given reactants are trichloroacetaldehyde, CCl3CHO and chlorobenzene. Chlorobenzene being more electron rich attacks the electron deficient carbon center of the carbonyl group of trichloroacetaldehyde. After that a removal of proton occurs then sulphuric acid which is a dehydrating agent removes a molecule of water to form a carbocation. Another molecule of chlorobenzene attacks the carbocation and finally with the removal of a proton product is formed.
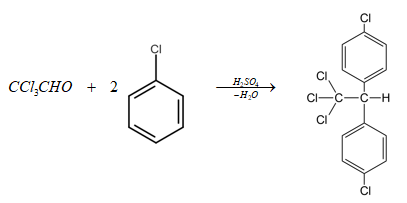
Additional Information: This reaction is a method of preparation of DDT, hence the final product is Dichlorodiphenyltrichloroethane commonly known as DDT and its IUPAC name is 1−chloro−4−[2,2,2−trichloro−1−(4−chlorophenyl)ethyl]benzene.
DDT, is an organochlorine and is a colourless, tasteless, and almost odourless crystalline
chemical compound. It was originally developed as an insecticide, but it became infamous for its environmental impacts. DDT was first synthesized by Othmar Zeidler while DDT's insecticidal action was discovered by Paul Hermann Müller . It was used to limit the spread of the insect borne diseases like malaria and typhus among civilians and troops in the second half of World War II .
Hence the correct answer is (iv).
Note: You must know the structure of the reagents given in the question for this kind of question otherwise proceeding with the reaction will become difficult. Also the options given are very similar in their structures and hence you must check the options correctly otherwise it may lead to error.
