Question
Question: Which is correct about above reaction?...
Which is correct about above reaction?
C-N coupling reaction: Carbanion is intermediate.
N-N coupling reaction: Carbocation is intermediate.
C-N coupling reaction: Carbocation is intermediate.
N-N coupling reaction: Carbanion is intermediate.
C-N coupling reaction: Carbocation is intermediate.
Solution
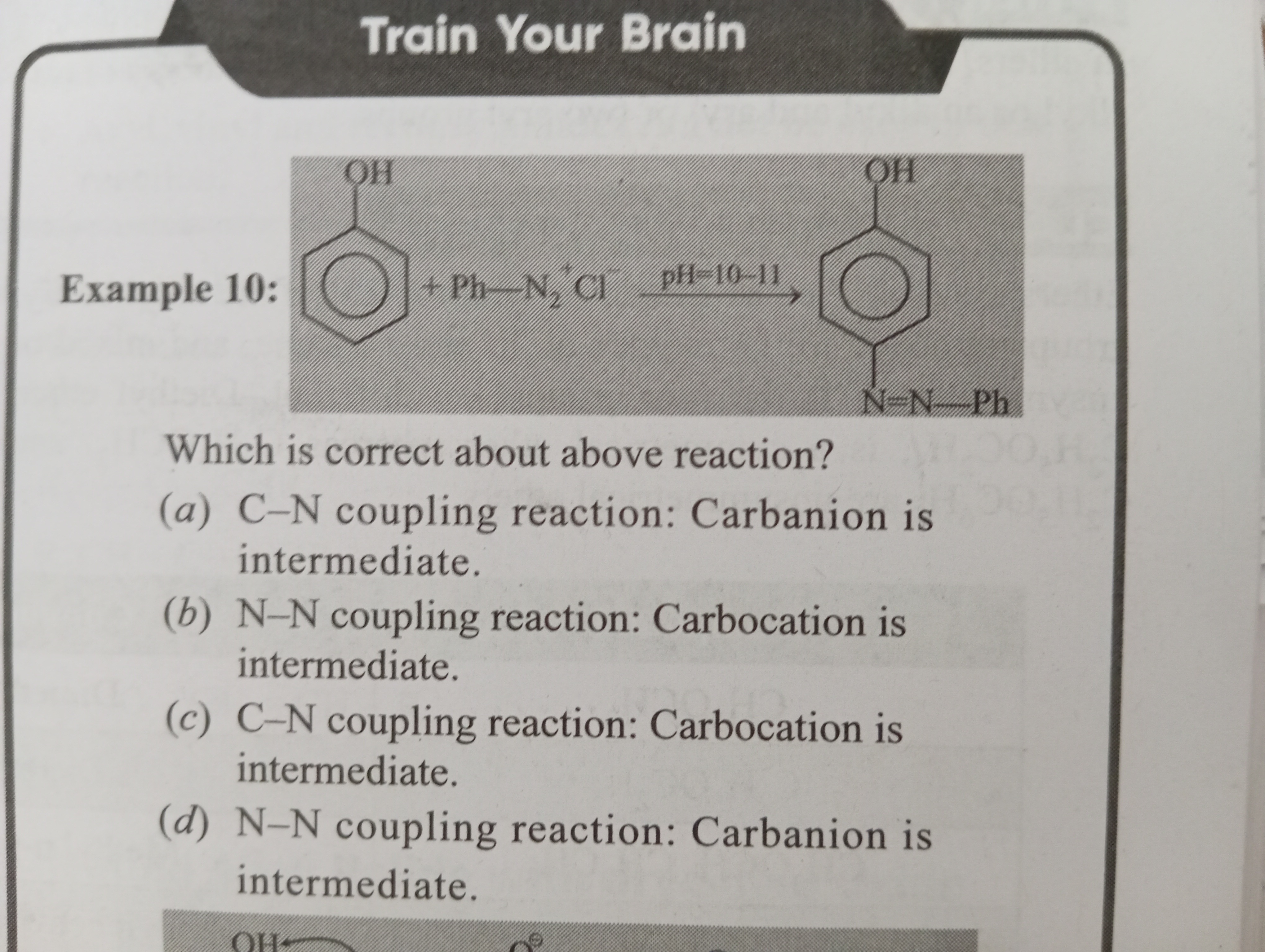
The given reaction is an azo coupling reaction between phenol and benzenediazonium chloride in an alkaline medium (pH 10-11).
-
Nature of Reactants in the given conditions:
-
Phenol (C₆H₅OH): In an alkaline medium (pH 10-11), phenol, being acidic, deprotonates to form the phenoxide ion (C₆H₅O⁻).
C₆H₅OH + OH⁻ ⇌ C₆H₅O⁻ + H₂OThe phenoxide ion is a much stronger activating group than phenol itself due to the negative charge on the oxygen. This negative charge delocalizes into the benzene ring, significantly increasing the electron density at the ortho and para positions, making the ring highly nucleophilic.
-
Benzenediazonium chloride (Ph-N₂⁺Cl⁻): The benzenediazonium ion (Ph-N₂⁺) acts as a weak electrophile.
-
-
Type of Reaction:
The reaction is an electrophilic aromatic substitution. The electron-rich phenoxide ring acts as the nucleophile, and the benzenediazonium ion acts as the electrophile.
-
Coupling Site and Product:
The coupling typically occurs at the para position to the -O⁻ group (if available) because it is sterically less hindered than the ortho positions and benefits from the strong activating effect of the -O⁻ group. The product formed is 4-hydroxyazobenzene (p-hydroxyazobenzene), which has a new bond formed between a carbon atom of the phenol ring and a nitrogen atom of the diazonium group. Therefore, this is a C-N coupling reaction.
-
Nature of the Intermediate:
In electrophilic aromatic substitution reactions, the attack of the aromatic ring (nucleophile) on the electrophile leads to the temporary loss of aromaticity and the formation of a resonance-stabilized intermediate known as a sigma complex or arenium ion. This intermediate carries a positive charge on the ring, making it a type of carbocation. Subsequently, this carbocation loses a proton (H⁺) from the carbon that was attacked, restoring aromaticity and forming the final product.
The mechanism can be visualized as:
- The electron-rich para carbon of the phenoxide ion attacks the terminal electrophilic nitrogen of the benzenediazonium ion.
- This forms a new C-N bond and generates an arenium ion (a resonance-stabilized carbocation).
- Deprotonation of the arenium ion restores aromaticity and yields the azo product.
Therefore, the intermediate formed is a carbocation.
Combining these points:
- The reaction is a C-N coupling reaction.
- The intermediate formed is a carbocation.
