Question
Question: Total right angles \(\angle Cl\,P\,Cl\) present in \(P\,C{l_5}\),\(P\,C{l_4}^ + ,P\,C{l_6}^ - \) are...
Total right angles ∠ClPCl present in PCl5,PCl4+,PCl6− are ______ respectively
a)0,1,4
b) 6,0,4
c) 2,4,0
d)6,0,12
Solution
When two lines are perpendicular to each other or intersect each other at 90∘, it is known as right angle. So draw the figures of each structure and try to find the number of right angles present.
Complete step by step answer:
PCl5 has a shape of Trigonal Bi Pyramidal geometry with a molecule of sp3d. Total valence electron present in PCl5 are:
PCl5 → Cl in group 17 so 5×7=35 and P is in group 15 so it has 5 electrons
Total electrons =35+5=40V.E.
Now Phosphorous is going to be in center as it can hold a lot of bonds.
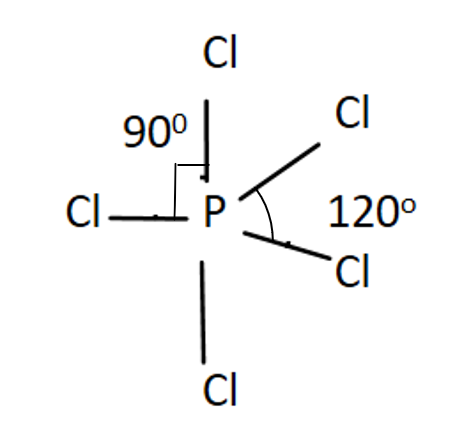
Five P-Cl bonds are formed.
There are 3 bonds of P−Cl which are at an angle of 120∘ and thus are known as equatorial bonds.
Other 2 P−Cl bonds are called axial bonds as these are at right angles to the equatorial bonds, i.e. one above and one below the plane.
So now there are 2 axial bonds where each of them are at right angles to 3 equatorial bonds thus giving 6 right angles. But ∠ClPCl are 2, so its answer is 2
PCl4+- It has a tetrahedral shape of tetrahedral. The oxidation state of P in PCl4+ is 5. Total valence electrons present in PCl4+ is
PCl4+ →Cl has 4×7=28V.E. and P has 5V.E.
Total valence electrons=5+28−1, this minus one is due to presence of negative sign on top.
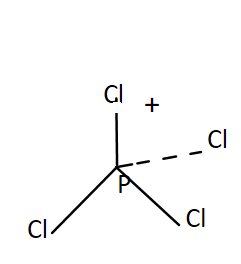 OR
OR 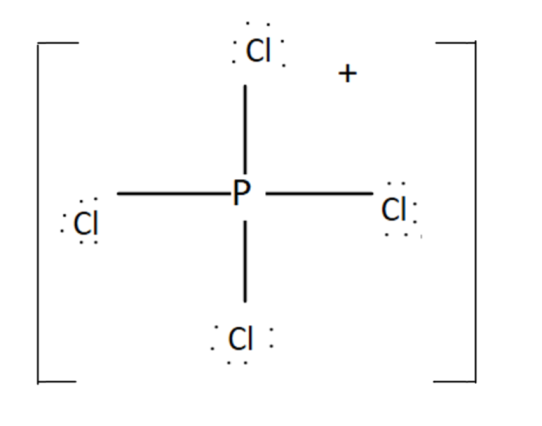
It has 4 bonding pairs and no lone pair. The bonds are pointing towards the corners of a regular tetrahedron. It was sp3 hybridisation. Thus PCl4+ has four right angles ∠ClPCl.
PCl6−- It has octahedral structure and sp3d2 hybridization.
For PCl6− we have +8 valence electrons (5+(6×7)+1)
Structure for PCl6− is as follow
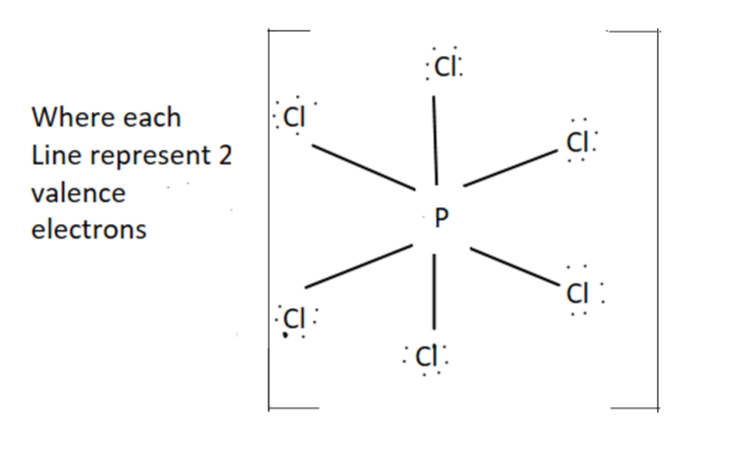
Hence we can see from the shape there is no right angle present between ∠ClPCl. So it has zero right angles.
So correct option is C
PCl5 has 2∠ClPCl right angles
PCl4+ has 4∠ClPCl right angles
PCl6− has 0∠ClPCl right angles.
Note: As we all know that bond angles are the geometric angles between two adjacent bonds. The molecular structure and the bond angles are dependent on each other. The atoms except the central atom of molecule adjust themselves in such a way so that they can feel less repulsion from each other and to do so they acquire maximum bond angles as much as possible.
