Question
Question: Total number of yellow precipitates which is/are soluble in yellow ammonium sulphide (YAS). CdS, Bi₂...
Total number of yellow precipitates which is/are soluble in yellow ammonium sulphide (YAS). CdS, Bi₂S₃, SnS, SnS₂, As₂S₃, As₂S₅, Sb₂S₃, Sb₂S₅, CuS, HgS
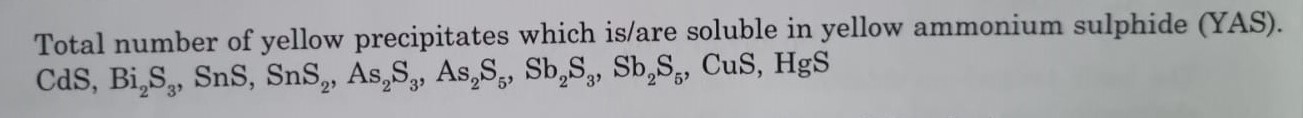
2
3
4
5
3
Solution
The given list of precipitates is: CdS, Bi₂S₃, SnS, SnS₂, As₂S₃, As₂S₅, Sb₂S₃, Sb₂S₅, CuS, HgS.
First, identify the yellow precipitates from the list:
- CdS: Yellow
- Bi₂S₃: Black
- SnS: Brown/Dark Brown
- SnS₂: Yellow
- As₂S₃: Yellow
- As₂S₅: Yellow
- Sb₂S₃: Orange
- Sb₂S₅: Orange/Red
- CuS: Black
- HgS: Black
The yellow precipitates are CdS, SnS₂, As₂S₃, and As₂S₅.
Second, determine which of these yellow precipitates are soluble in yellow ammonium sulphide (YAS). In qualitative analysis, sulfides of Group IIB elements (As, Sb, Sn) are soluble in YAS, while sulfides of Group IIA elements (Cu, Cd, Hg, Bi) are insoluble in YAS.
Let's examine the solubility of the yellow precipitates in YAS:
- CdS: Cadmium (Cd) is a Group IIA element. CdS is insoluble in YAS.
- SnS₂: Tin (Sn) is a Group IIB element. SnS₂ is soluble in YAS, forming soluble thiostannate: SnS₂ + (NH₄)₂Sₓ → (NH₄)₂[SnS₃] + (x-1)S (or other thiostannate species).
- As₂S₃: Arsenic (As) is a Group IIB element. As₂S₃ is soluble in YAS, forming soluble thioarsenite/thioarsenate: As₂S₃ + (NH₄)₂Sₓ → (NH₄)₃[AsS₃] + (x-1)S (or other thioarsenate species).
- As₂S₅: Arsenic (As) is a Group IIB element. As₂S₅ is soluble in YAS, forming soluble thioarsenate: As₂S₅ + (NH₄)₂Sₓ → (NH₄)₃[AsS₄] + (x-1)S (or other thioarsenate species).
The yellow precipitates that are soluble in yellow ammonium sulphide (YAS) are SnS₂, As₂S₃, and As₂S₅.
The total number of such precipitates is 3.
