Question
Question: Total number of \(\sigma \)bonds in \({{N}_ {3}}H\)is _______...
Total number of σbonds in N3His _______
Solution
Hydrazoic acid, also known as hydrogen azide or azoimide, is a compound with the chemical formula N3H. It is a colourless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride.
Complete step by step solution:
We have been provided with a compound N3H,
Its IUPAC name Hydrogen azide is a nitrogen hydride. It is a conjugate acid of an azide anion,
The hybridisation of the central N atom is only sp. And, remaining two N atoms is sp2
Its structure is:
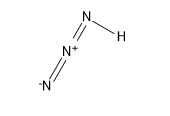
Sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups.
Pi bonds are covalent chemical bonds where two lobes of an orbital on one atom overlap two lobes of an orbital on another atom and this overlap occurs laterally. Each of these atomic orbitals has zero electron density at a shared nodal plane, passing through the two bonded nuclei.
From this we came to know that Hydrazoic acid has 3 sigma bonds with 3 hydrogens and zero pi bonds and has a lone pair of electrons.
Note: N3H is called the covalent azide and it does not dissociate easily. This makes sodium azide more stable than hydrazoic acid. Hence, Na+N3−decomposes at a much higher temperature than N3Hand hence, the higher thermal stability of NH3Na.
