Question
Question: Total number of geometrical isomers for the following compound is 
A.8
B. 2
C. 4
D. 3
Solution
We can define isomers as the compounds with the same molecular formula but differ in the arrangement of the atom. Stereoisomers are the isomers that have the same molecular formula but differ in spatial arrangement of atoms. Stereoisomers can be subdivided into two groups,
-Optical isomers
-Geometrical isomers
Complete step by step answer:
We can define geometrical isomers of atoms having different arrangements on either side of a double bond above or below the ring of a cycloalkane or cycloalkane. If the atoms are present on the same side of the double bond, then it is cis-isomer and if they are present on the opposite side of the double bond, then it is trans-isomer.
If there is restricted rotation in a molecule then it is geometric isomerism. Geometric isomers are also known as Cis- trans isomerism.
The given compound is,
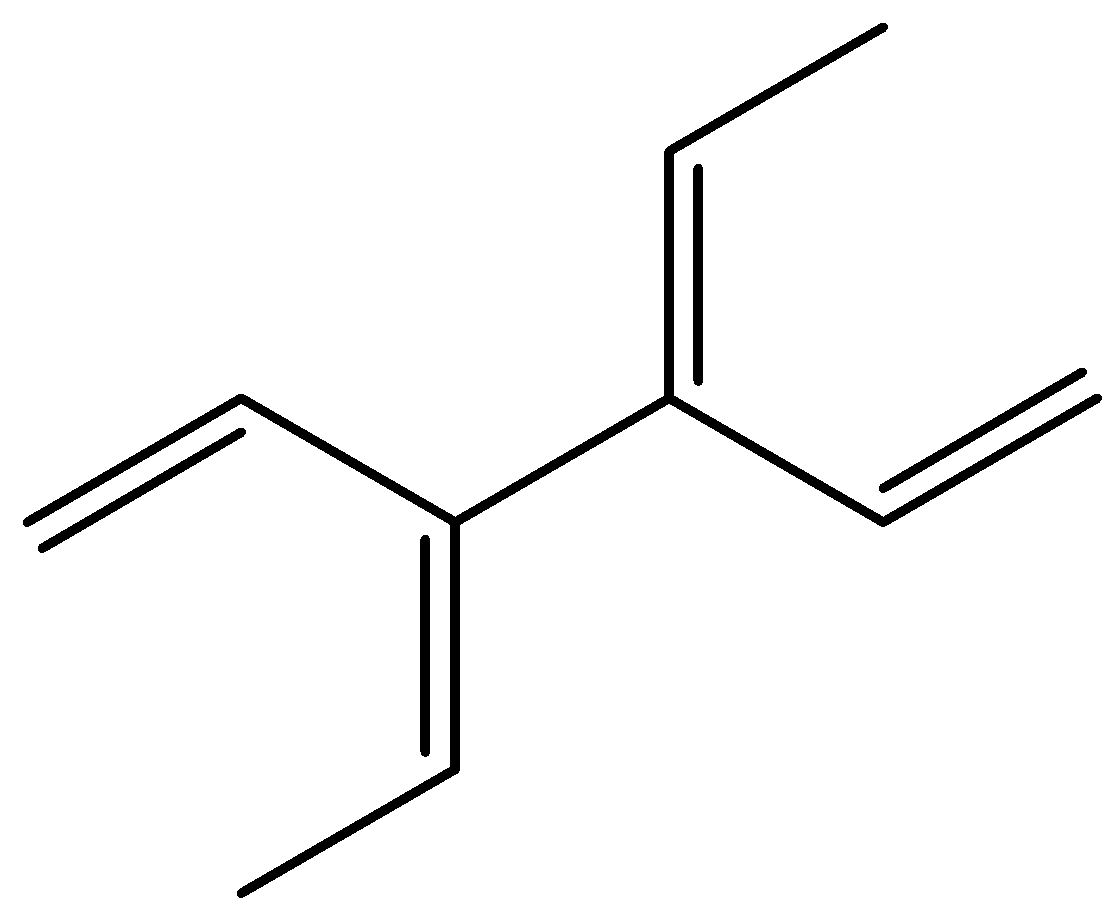
The total number of geometrical isomers in the given compound is four.
Hence option C is the correct answer.
Additional information:
As we have studied, isomers are molecules with the same molecular formula. Isomerism is the spatial arrangement of atoms having the same molecular formula but differ in physical and chemical properties.
There are two types of isomerism, one is structural isomerism and other is stereoisomerism.
In structural isomerism the isomers have the same molecular formula but their structure is different.
E.g., Chain isomerism, position Isomerism.
In stereoisomerism, the molecules have the same molecular but differ in spatial arrangement. E.g., Geometrical isomerism and optical isomerism.
Note:
We can write skeletal structures with crosses lines for bonds to represent geometrical isomers. Though the IUPAC name does not recommend the crossed line notation, wavy lines linking a double bond to a heteroatom are preferred. Cis-and trans- are given as prefixes to chemical structures.
