Question
Question: Total number of geometrical isomers for the complex \(\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{...
Total number of geometrical isomers for the complex [RhCl(CO)(PPh3)(NH3)] is: __
Solution
The isomerism in which the position of the ligands in the space differs is known as geometrical isomerism. The square planar complexes show geometrical isomers. These complexes can be of various types such as MA2B2 , MA2BC , MABC2 ,MABCD . The number of isomers depends on the number of different positions acquired by the ligand.
Complete answer:
Geometrical isomerism arises in heteroleptic complexes due to ligands occupying different positions around the central ion. The ligands occupy the positions either adjacent to one another. These are referred to as cis-form (ligands occupy adjacent positions) and transforms (ligands occupy opposite positions). This type of isomerism is, therefore also referred to as the cis-trans isomerism.
This type of isomerism is very common in coordination compounds. This is due to different coordination numbers varying from 2 to 9, commonly encountered in these compounds.
We have given the coordination compound of ruthenium [RhCl(CO)(PPh3)(NH3)] . It is coordination compounds, such that the central atom Rh is bonded to four different ligands: Cl , CO , PPh3 and NH3 .
Since Rh is bonded with four ligands, it must be a square planar complex. The bonded groups are different thus its general representation is, MABCD , where A, B, C, and D are ligands.
The square planar complexes of MABCD type structure show three isomers. the structures of these isomers can be written by fixing a position of a ligand (let say A) and placing other and D ligands trans to it.
To determine the geometrical isomers of [RhCl(CO)(PPh3)(NH3)] let's fix the position of Cl the ligand and place the other ligands trans to it. The geometrical isomers for the [RhCl(CO)(PPh3)(NH3)] are as shown in the figure.
Thus, here we conclude that the coordination complex [RhCl(CO)(PPh3)(NH3)] has three isomers.
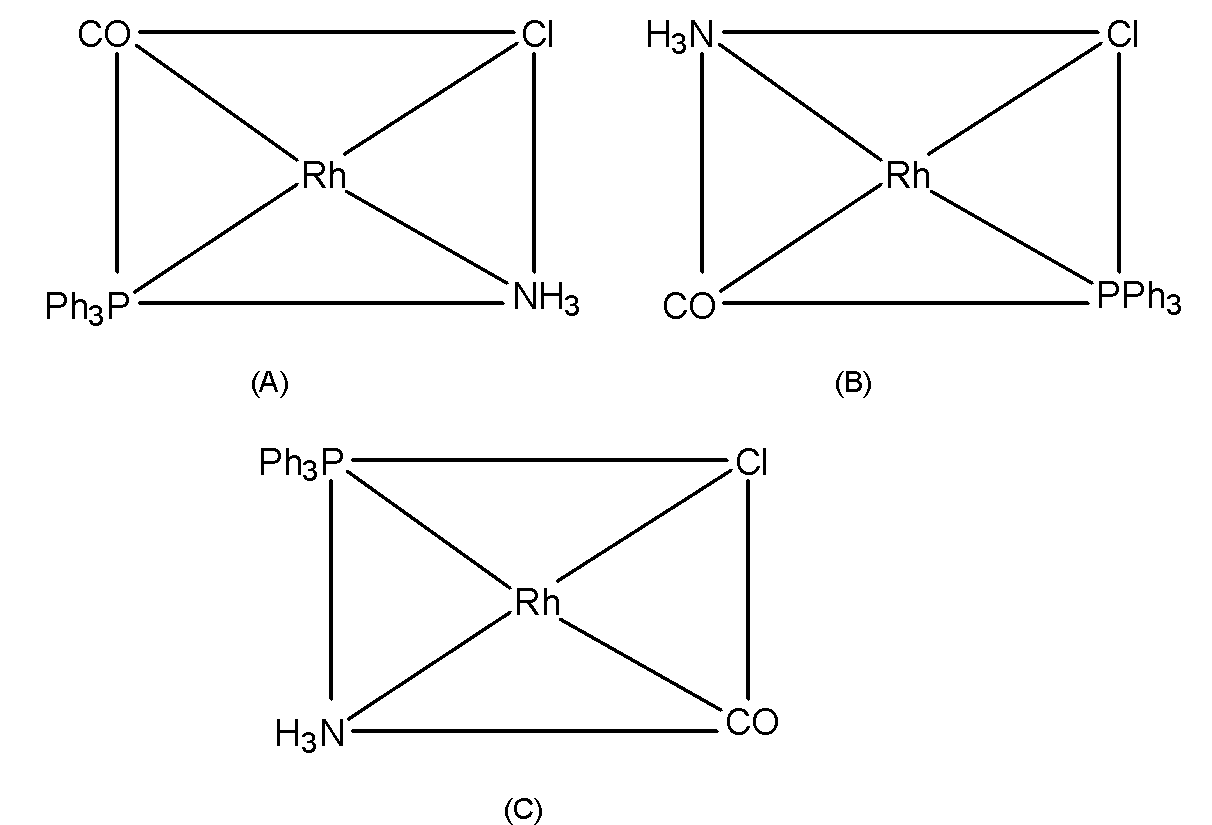
Here, (A), (B), and (C) are geometrical isomers of the complex.
Note:
Note that, we can draw forth geometrical isomers.IN which the Chloride ligand is trans to the PPh3 ligand which is identical to the (A) form. Thus, only three isomers are possible for the MABCD type of complex. The geometrical isomers are not possible in tetrahedral complexes as all the positions are adjacent to each other in these complexes. Thus, geometrical isomerism is strictly related to the square planar complexes.
