Question
Question: Total number of cis N-Mn-Cl bond angles (that is, Mn-N and Mn-Cl bonds in cis-position) present in a...
Total number of cis N-Mn-Cl bond angles (that is, Mn-N and Mn-Cl bonds in cis-position) present in a molecule of cis [Mn(en)2Cl2]complex is ---? (en = NH2CH2CH2NH2).
Solution
The given molecule cis [Mn(en)2Cl2]is a coordination complex with manganese as the central metal atom. As mentioned above, en represents the compound ethylene diamine which is a bidentate ligand.
Complete answer:
-A bidentate ligand has two centers of attachment to bond with the central metal atom. So ethylene diamine gets attached to the central metal by forming two bonds between two nitrogen atoms with the manganese atom. As there are two en molecules each of which forms two Mn-N bonds and two chlorine atoms form two Mn-Cl bonds which form six bonds in total.
-Cis [Mn(en)2Cl2] contains two en ligands lying on same side of the plane and two chlorine atoms lying on the adjacent on the other side. Upon arranging all the bonds that the ligands form with the metal, two Mn-Cl and four Mn-N in 3dimensional space, the complex attains the structure of octahedron. Octahedral arrangement of cis- [Mn(en)2Cl2]shows six N-Mn-Cl bond angles in total. The structure of cis [Mn(en)2Cl2] is shown below to understand the bonds in it and how it contains six N-Mn-Cl bond angles.
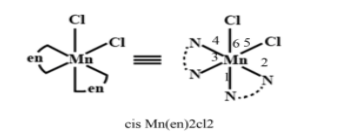
Therefore the answer for the above question is six (6).
Note: Cis and trans notations are used for the two structural isomers of a complex compound where cis isomer has the same ligands attached on same side and the in trans form, same ligands are attached on opposite sides of the metal atom.
