Question
Question: To prepare propan-2-ol from methyl magnesium iodide, the chemical reagent required is: A.\[C{H_3}C...
To prepare propan-2-ol from methyl magnesium iodide, the chemical reagent required is:
A.CH3CHO
B.HCHO
C.CH3COCH3
D.CO2
Solution
We must have to know that the propan-2-ol, otherwise known as isopropyl alcohol, is a chemical compound having the formula C3H8O. Here the isopropyl group is attached with a hydroxyl group and there is a formation of secondary alcohol. And the alcohol carbon atom is linked with two different carbon atoms. The propan – 2- ol is mainly used for the preparation of solvents like dye solutions, soap, etc.
Complete answer:
When the acetaldehyde is reacting with methyl magnesium iodide, there is a formation of secondary alcohol which is propan – 2 – ol.
Here the methyl magnesium iodide is linked with carbonyl carbon and there is a formation of adduct. And due to the electronegativity difference, adducts have partial charges. And thus adduct is reacted with water and undergoes the hydrolysis, will get propan – 2 – ol.
Let’s see the reaction,
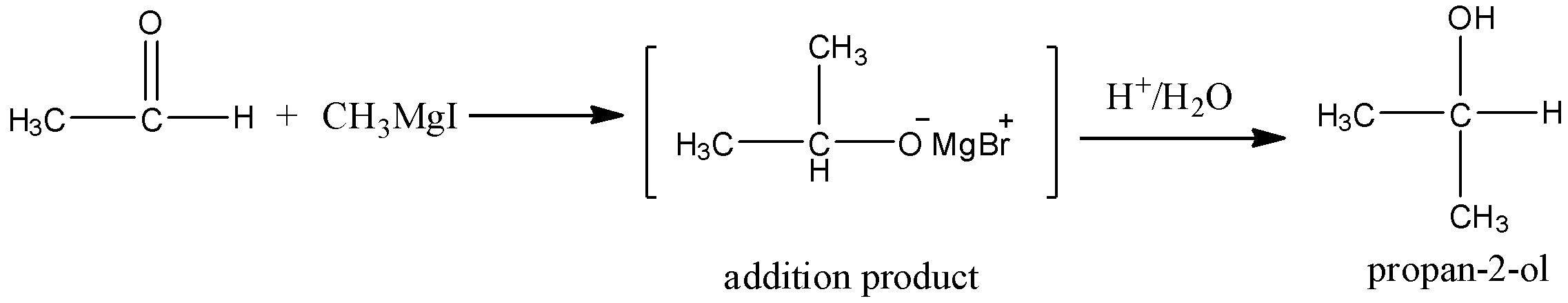
Hence, option (A) is correct.
When the formaldehyde is reacting with methyl magnesium iodide, (Grignard reagent), there is a formation of primary alcohol. But here, propan -2- ol is a secondary alcohol. Hence, the option (B) is incorrect.
When acetone is reacting with methyl magnesium iodide, there is a formation of 2 -methyl 2 propanol and will not get propan – 2 – ol. Hence, option (C) is incorrect.
When the carbon dioxide is reacting with methyl magnesium bromide, the product should be a carboxylic acid and not give propan – 2 – ol. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We need to know that the Grignard reagent is a chemical compound which is widely used as a reagent in organic synthesis. The general formula of Grignard reagent is, R−Mg−X. If the Grignard reagent is reacted with aldehyde or ketone, there is a formation of secondary alcohol and tertiary alcohol respectively. But, when the formaldehyde is reacting with Grignard reagent, there is a formation of primary alcohol.
