Question
Question: To compare the stability of the given compoundThe stability of carbanions in the following is in the...
To compare the stability of the given compoundThe stability of carbanions in the following is in the order of:
a.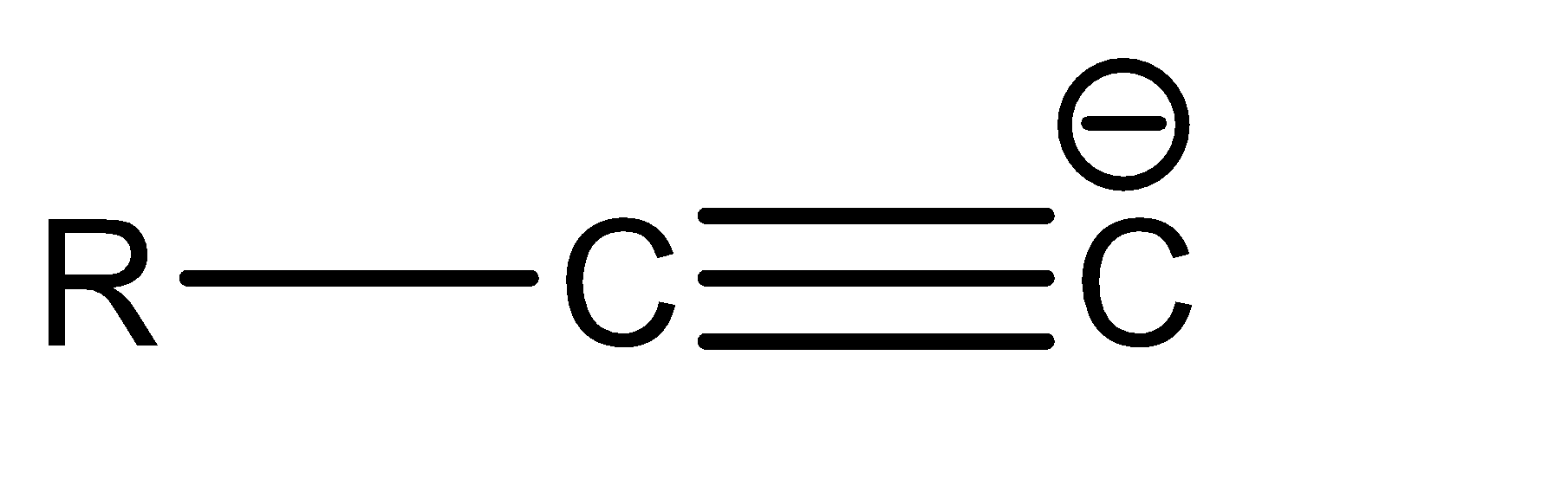
b.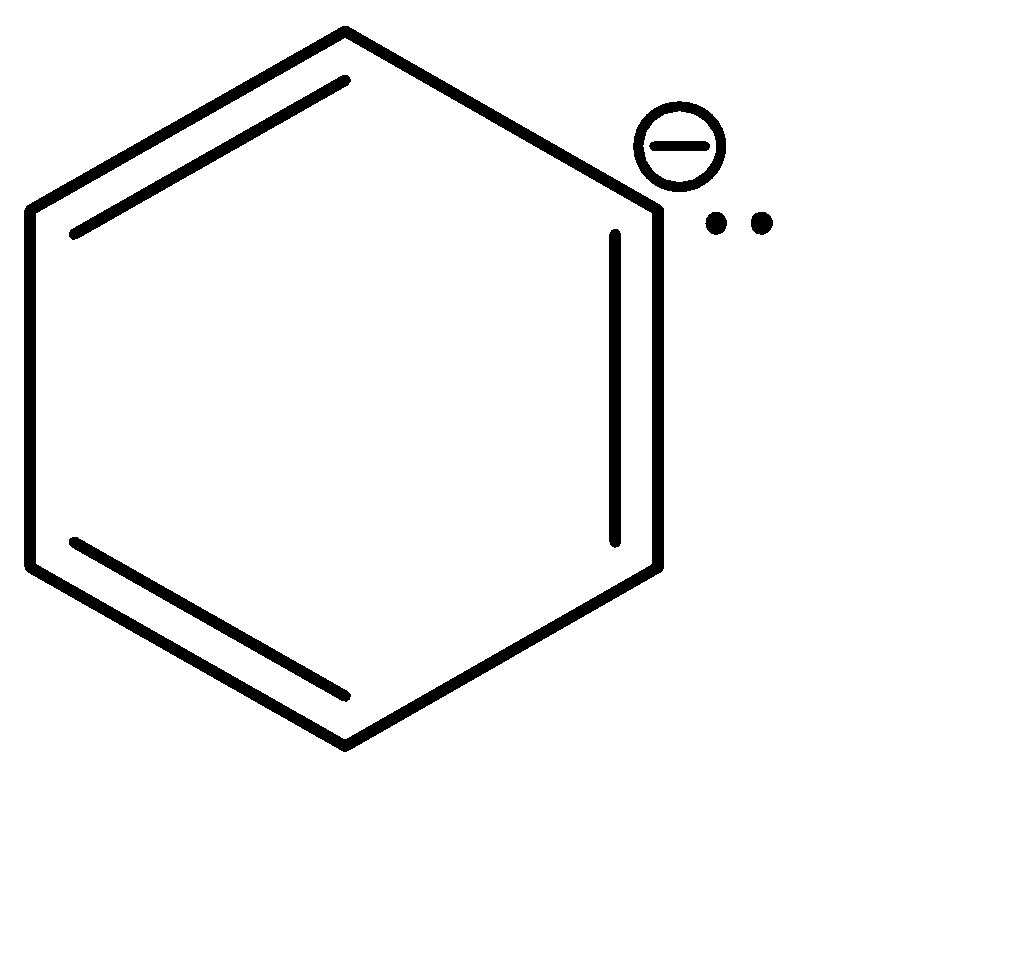
c.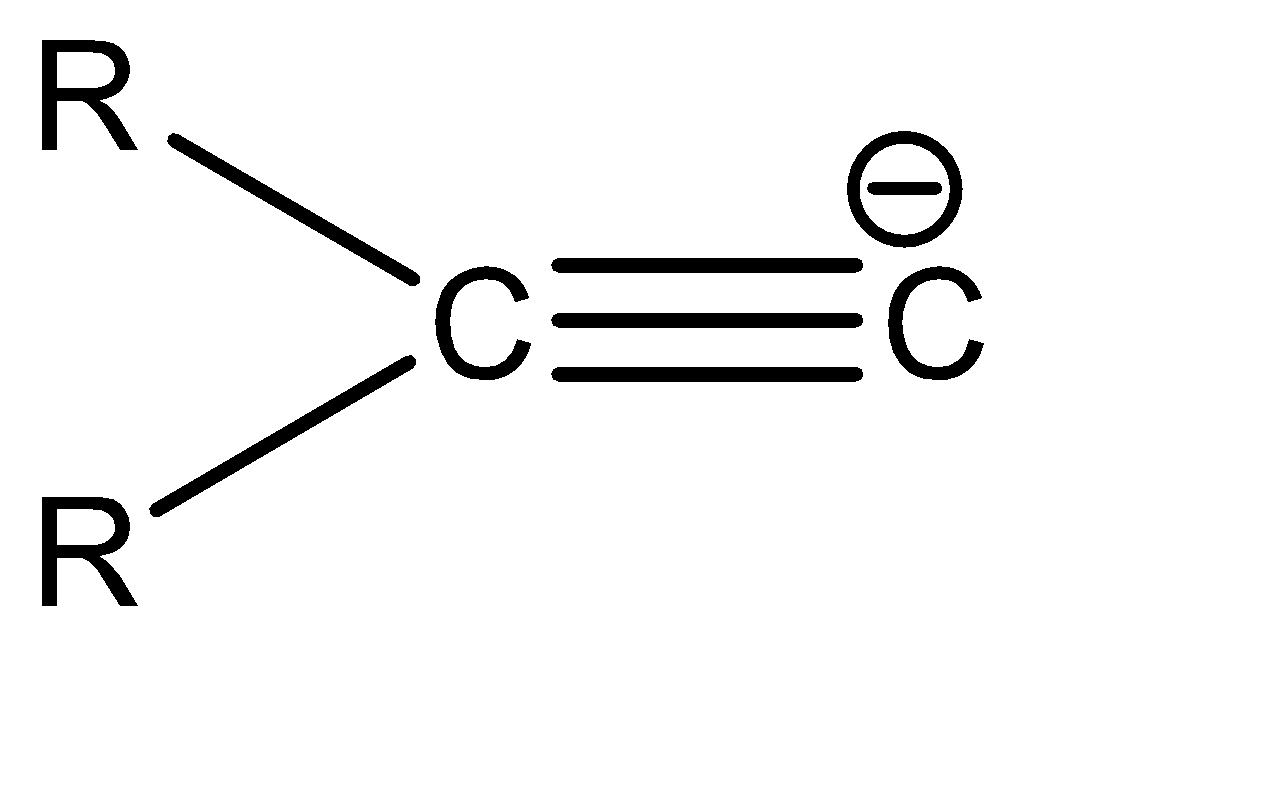
d.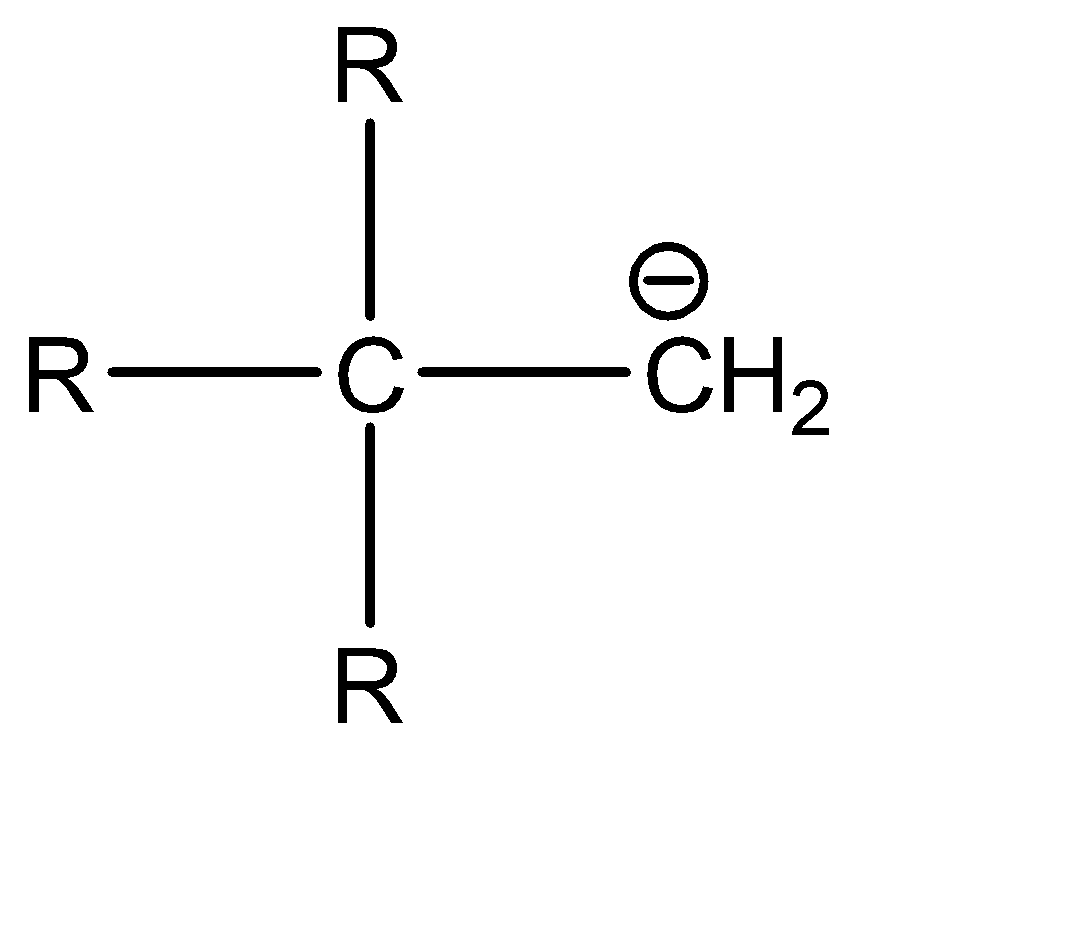
A.a > c > b > d
B.a > b > c > d
C.b > c > d > a
D.d > b > c > a
Solution
Carbanions can be understood as the anions of carbon. This means that carbanions are formed when a carbon atom has an unshared pair of electrons, due to which it bears a negative charge usually with three substituents for the total number of electrons to be 8 electrons. The geometry of a carbanion is pyramidal. Carbanions can also be explained as conjugate bases of carbon acids.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The stability of a carbanion is dependent on several factors. They are stated as follows:
1.Inductive effect: an increase in the positive inductive effect of the adjacent substituent atoms increases in the stability of the carbanion. One of the important substituents in a carbanion is the alkyl group. Alkyl group exhibits + I effect and tends to release electrons towards the charged carbon. Hence, higher the number of alkyl groups adjacent to the charge carbon, higher is the stability of the carbanion.
2.If the charge bearing carbon atom is hybridized, then the stability of carbanion would also depend on the s character of the charge bearing carbon. Higher the s character, higher would be the stability.
3.Resonance effect: if the carbanion exhibits resonance, then it is more stable than regular carbanions. If all the carbanions under consideration exhibit resonance, then the one with a greater number of conjugated structures is more stable.
| Compound| Hybridization of charged carbon
---|---|---
a| 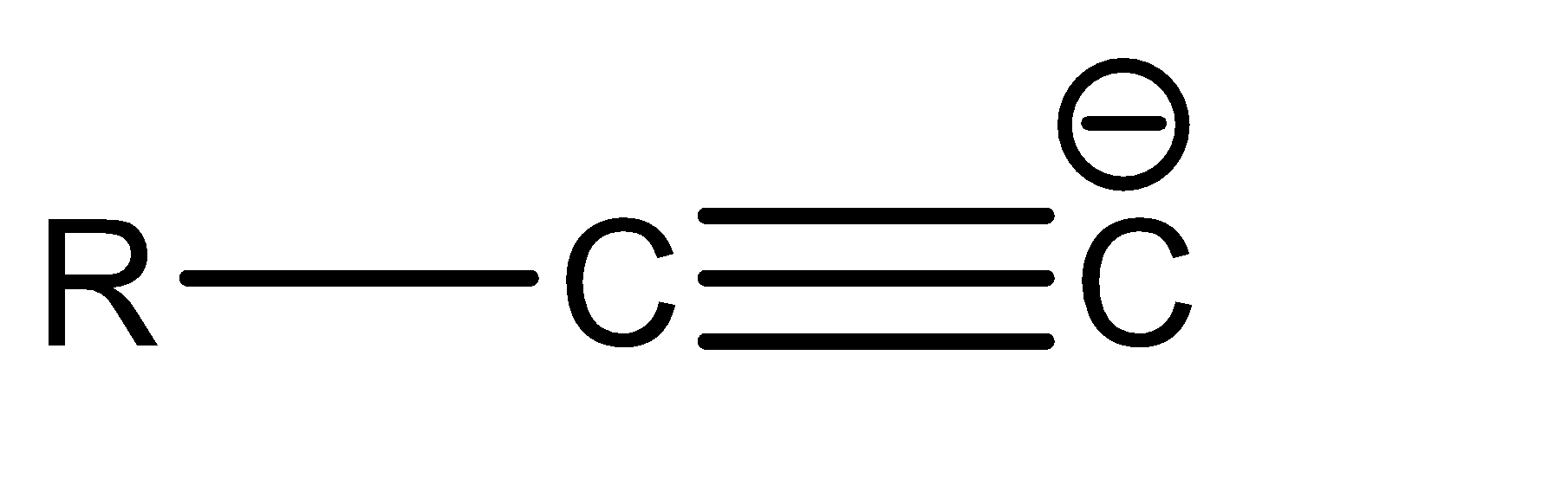 | sp
| sp
b| 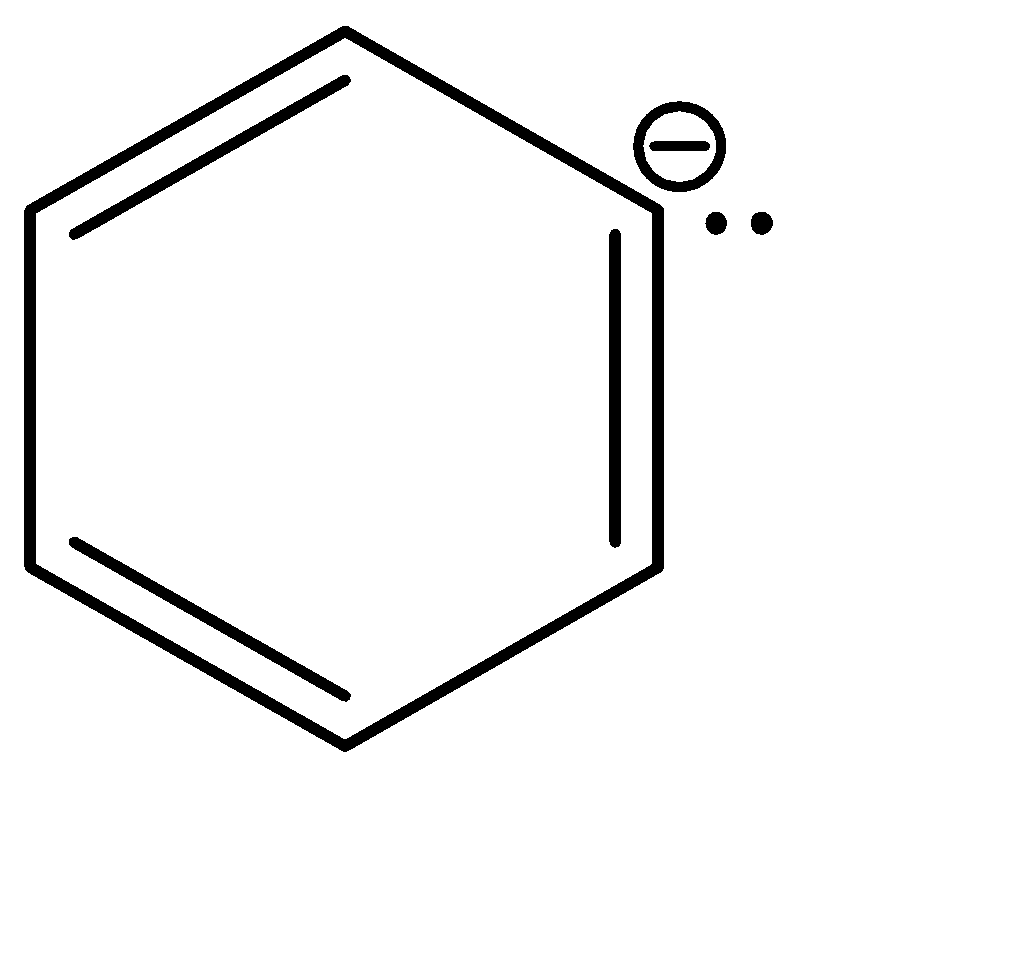 | sp2
| sp2
c| 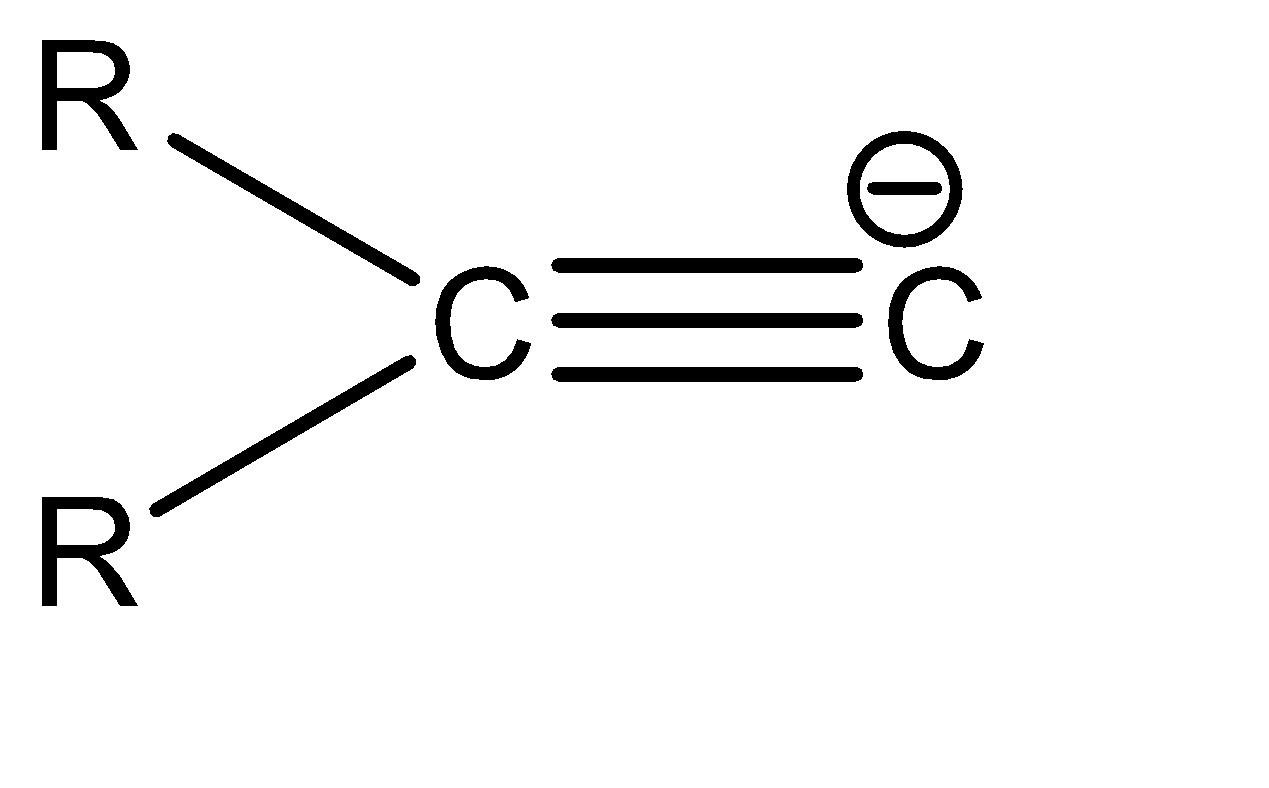 | sp2
| sp2
d| 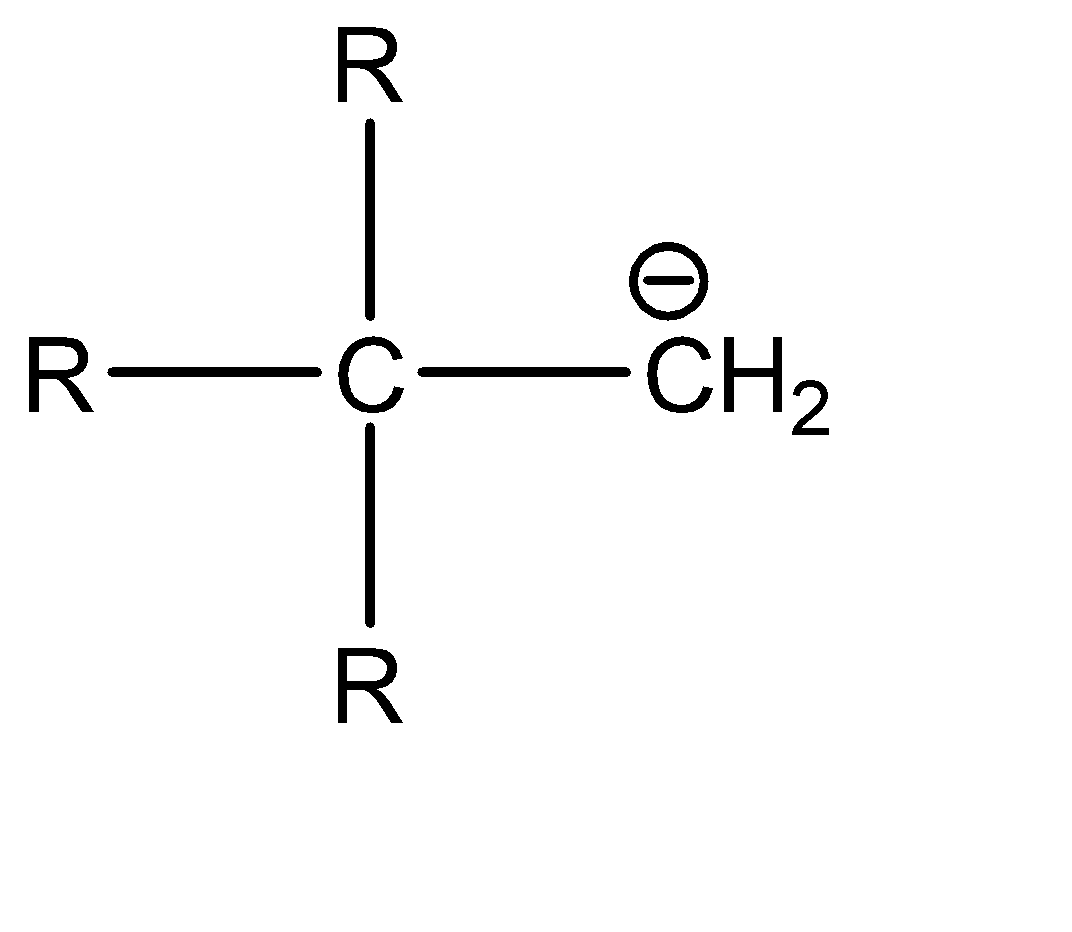 | sp3
| sp3
Now, since compound b and c have the same hybridization, they can be compared on the basis of the delocalisation of the electron cloud. The delocalisation is greater in option b, which in turn makes it more stable. Hence, the correct order of stability is: a > b > c > d
Hence, Option B is the correct option
Note: The alkyl group does not literally "push" the electrons away - the other end of the bond attracts them more strongly. This means that the alkyl group becomes slightly positive (+) and the carbon they are attached to becomes slightly negative (-).
