Question
Question: Titration curves for \(0.1{\text{M}}\) solutions of three weak acids \({\text{H}}{{\text{A}}_1},{\te...
Titration curves for 0.1M solutions of three weak acids HA1,HA2,HA3 with ionization constants K1,K2,K3 respectively are plotted in the figure. Which of the following is/are true?
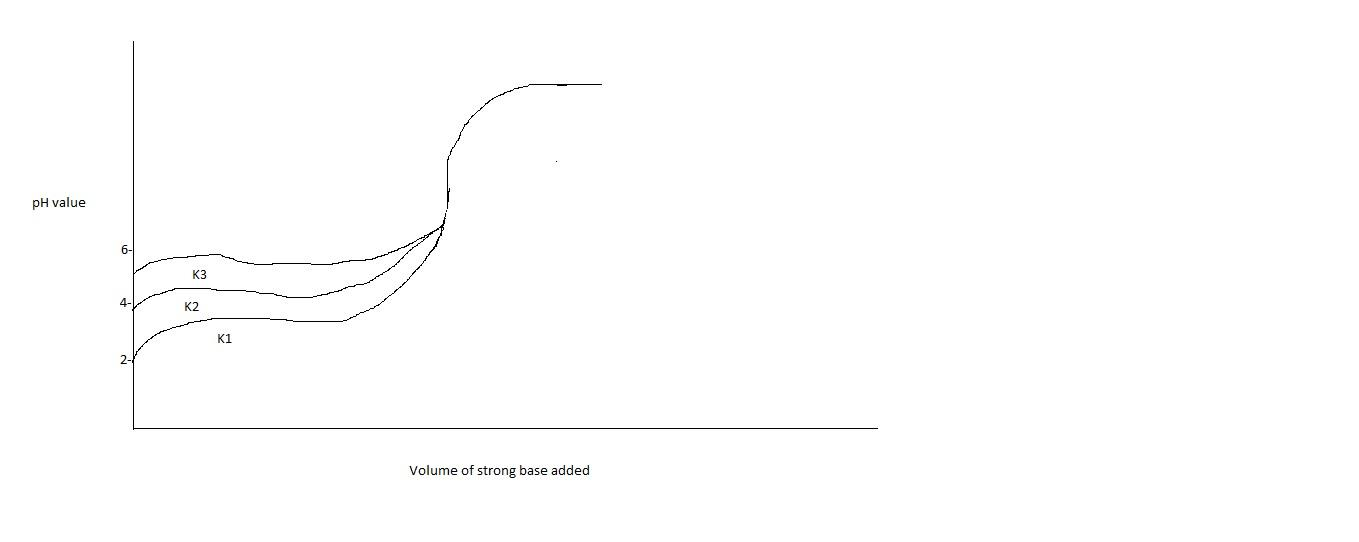
A. K2=2(K1+K3)
B. K1<K3
C. K1>K2
D. K2>K3
Solution
Ionization constant of an acid is the equilibrium constant for the reaction of a weak acid with water. Similarly the ionization constant of a base is the equilibrium constant for the reaction of a weak base with water. Acid strength increases with ionization constant.
Complete step by step answer:
Acids are generally defined as the hydrogen containing compounds and bases are the hydroxide containing compounds. Ionization is the process in which individual positive and negative ions are produced from a molecular compound that is dissolved in a solution. While dissociation is the process in which positive and negative ions are released from an ionic compound that is dissolved in a solution. Weak acids transfer only a small percentage of its protons to water in aqueous solution.
In the titration curve of weak acids, only a fraction of weak acid is dissociated. Before adding the base, pH of the weak acid is greater than that of strong acid. Initially the pH changes rapidly. At an equivalence point (the point at which neutralization is complete), the solution becomes basic. When the acid is neutralized, pH is influenced by the addition of base in excess. Ionization constant is the equilibrium constant. When ionization constant is increased, pH is increased.
Thus from graph, we can tell that K1 has less value than K2 and K3. Moreover, K2 is in the middle of K1 and K3. Therefore option A will be correct. K2 will be the average of K1 and K3. Moreover, option B is also correct, i.e. K1<K3.
Thus options A and B are correct.
Additional information:
pH is calculated from the value of [H3O+]. It is the negative algorithm of hydronium ion concentration. In pure water hydronium concentration is the same as that of hydroxide ion concentration.
Note:
In any acid-base reaction, the equilibrium will favor the reaction that moves the proton to the stronger base. When HCl is used, since water is stronger than Cl−, equilibrium moves to the right. During auto-ionization, the ionization constant is called the ion-product constant.
