Question
Question: Thyroxine, the hormone has given below structure The percentage of iodine in thyroxine is ____%. (n...
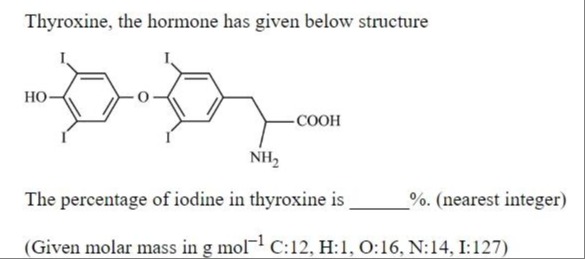
Thyroxine, the hormone has given below structure
The percentage of iodine in thyroxine is ____%. (nearest integer)
(Given molar mass in g mol−1 C:12, H:1, O:16, N:14, I:127)
Answer
65%
Explanation
Solution
-
Determine the molecular formula:
\ceC15H11I4NO4
The structure described is thyroxine, which has the formula -
Calculate the molar mass:
M=15×12(C)+11×1(H)+4×127(I)+1×14(N)+4×16(O)=180+11+508+14+64=777g/mol -
Find the percentage of iodine:
%I=(777508)×100≈65.3%
Mass of iodine = 4×127=508gRounding to the nearest integer gives 65%.
Thyroxine’s molecular formula is \ceC15H11I4NO4. Its molar mass is calculated as 777 g/mol; iodine contributes 508 g. Thus, percentage iodine is (508/777)×100≈65%.
