Question
Question: Three very large plates of the same area are kept parallel and close to each other. They are conside...
Three very large plates of the same area are kept parallel and close to each other. They are considered as ideal black surface sand and have very high thermal conductivity. The first and the third plates are maintained at temperature 2T and 3T respectively. The temperature of the middle (i.e., second) plate under a steady state condition is:
A. (265)41T
B. (497)41T
C. (297)41T
D. (97)41T
Solution
Ideal black surfaces are the surfaces which can emit all the energy absorbed by the body. When a body absorbs energy from its surrounding the process is known as an endothermic process. In the endothermic process, the temperature of the body increases according to the energy absorbed.
Complete answer:
The temperature of plate 1 and two is 2T and 3T respectively.
When a plate (P2 ) is in the middle of the two plates (P1,P3) and all of them are of the same area and are parallel to each other. As shown below.
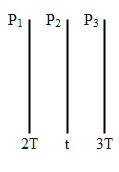
Under steady-state conditions, the rate of energy received by the middle plate will be equal to the energy radiated by the plate. Or in other words, the energy absorbed by the middle plate will be equivalent to the energy emitted by both plates 1 and 3.
Let, t be the temperature of the middle plate
A be the area of the plates
So the steady-state condition can mathematically be framed as,
