Question
Question: Three times at three different values of constant pressure are given. Which of the following relatio...
Three times at three different values of constant pressure are given. Which of the following relations is correct?
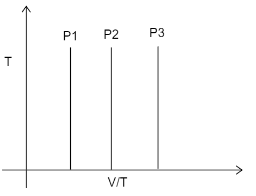
A) P1=P2=P3
B) P1>P2>P3
C) P3>P2>P1
D) Can be predicted
Solution
To solve this question, we will need to recall the ideal gas equation. The ideal gas equation is given as: PV=nRT
Where, P is the pressure (in atm), V is the volume (in Litres) , n is the total moles of the gas present in the mixture, R is the gas constant and T is the temperature (in Kelvin). The value of R changes with the given Pressure and Volume. The various values of R can be given as: 8.314J/K/mol,0.0821Latm/mol,2cal/Kmol . This equation is not applicable to Real Gases. If the nature of the gas is not specified, consider it as ideal only.
Complete answer:
In the graph we can see that all the three pressures are given at a constant temperature ‘T’ only. The graph given is T v/s V/T. According to the ideal gas equation: PV=nRT
From this equation we can say that P=VnRT . If we consider n and R to be constant, we get that P=VT , pressure is inversely proportional to V and directly proportional to T. we are given V/T, in which P is directly proportional to T and inversely proportional to V. The condition given to us is constant temperature (T). Hence as the value of V/T increases P will decrease.
We are given three pressures P1,P2,P3 . As the value V/T increases the pressure will decrease. The relationship will thus become, P1>P2>P3 .
The correct answer is Option B).
Note:
Alternatively, we can say that, V∝P1 . Since T is constant, V=T.P1 . This can be written as, TV∝P1 . Thus, the more the value of V/T lesser will be the value of P. The inverse relationship can also be established like this. The relationship between P1,P2,P3 will be the same.
