Question
Question: Three metal rods made of copper, aluminium and brass, each \(20cm\) long and \(4cm\) in diameter, ar...
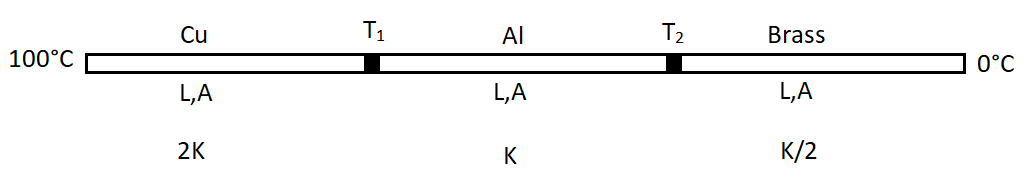
Three metal rods made of copper, aluminium and brass, each 20cm long and 4cm in diameter, are maintained at 100∘ and 0∘ respectively. Assume that the thermal conductivity of copper is twice that of aluminium and four times that of brass. The approximately equilibrium temperatures of copper-aluminium and aluminium-brass junctions are respectively.
(A) 68∘ and 75∘C
(B) 75∘ and 68∘C
(C) 57∘ and 86∘C
(D) 86∘ and 57∘C
Solution
Hint Using the formula for the heat current, we can equate the heat current in the three rods since they are connected in series. Then we can find the value of the equilibrium temperatures of the junctions from the equations.
Formula Used: In the solution we will be using the following formula,
H=LΔTKA
where H is the heat current flowing, ΔT is the difference in temperatures,
K is the thermal conductivity of the material, A is the area of cross-section,
and L is the length of the wire.
Complete step by step answer
According to the picture that is given in the question, we see that the three rods are connected in series with each other. So the heat current that is flowing through the three rods will be equal to one another. Now according to the question the length and the area of cross-section of all the three rods are the same. So we take them asL and A for all the rods. The thermal conductivity of copper is twice that of aluminium and four times that of brass. So we take the thermal conductivity of aluminium as K. So the thermal conductivity of copper is 2K and that of brass is 2K
Therefore we can use the formula for the heat current through each of the rods as,
HCu=L(100−T1)2KA
HAl=L(T1−T2)KA and
HBrass=2L(T2−0)KA
On equating the three we have,
L(100−T1)2KA=L(T1−T2)KA=2L(T2−0)KA
Now from HAl=HBrass
L(T1−T2)KA=2L(T2−0)KA
On cancelling the common terms on both the sides we have,
T1−T2=2T2
On taking the similar terms to one side we have,
T1=T2+2T2
On adding we have
T1=23T2
Now from HCu=HAl we get,
L(100−T1)2KA=L(T1−T2)KA
Again on cancelling the common terms we have,
2(100−T1)=T1−T2
On opening the brackets and taking common terms to one side we get,
200=2T1+T1−T2
Hence we have,
200=3T1−T2
Now on substituting T1=23T2 we get,
200=3×23T2−T2
On subtracting we get,
200=2(9−2)T2
Hence we get the temperature as,
T2=7200×2
On calculating this gives us,
T2=57.14∘C
This is approximately equal to T2≃57∘C
Substituting this value in T1=23T2 we get,
T1=23×57
Hence, T1=85.5∘C
This is approximately equal to,
T1≃86∘C
So the temperatures are 86∘ and 57∘C. So the correct option is D.
Note
The heat current is described as the rate of exchange of kinetic energies between two molecules. It can also be described as the rate of transfer of heat with respect to the time. It can be written in the form of H=dtdQ, where Q is the heat and t is the time.
