Question
Question: Three Centre two electron bond is present in: (A).\(N{{H}_{3}}\) (B).\({{B}_{2}}{{H}_{6}}\) ...
Three Centre two electron bond is present in:
(A).NH3
(B).B2H6
(C).BCl3
(D).AlCl3
Solution
In a three center two electron bond the two electrons are shared by three separate nuclei. .Elements that are able to form electron deficient bonds are involved.In banana bond, the probability of distribution of this bond is in shape of banana.
Complete answer:
- A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons.
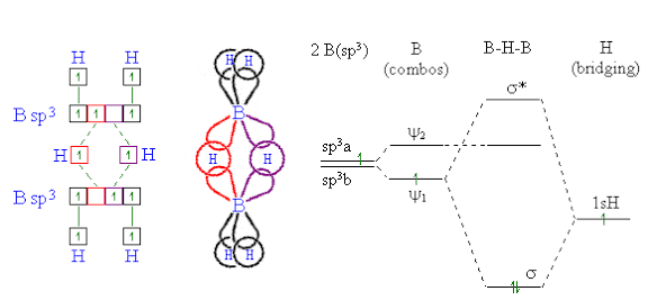
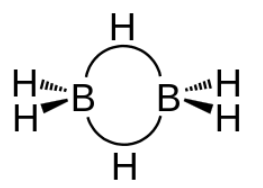
-As boron atom has an empty p-orbital so the monomer BH3 is unstable.when a boron atom shares electrons with a B−H bond on another boron atom, AB−H−B 3-center-2-electron bond is formed . across three internuclear spaces, the two electrons (corresponding to one bond) in a B−H−B bonding molecular orbital are spread out .In diborane (B2H6 ), two H atoms bridge the two B atoms, leaving two additional H atoms in ordinary B−H bonds on each .There are two such 3c−2e bonds: As each boron atom participates in a total of four bonds and there is filling of all bonding molecular orbitals and two of the four bonds are 3-centre B−H−B bonds, so the molecule is stable.
Option B is the correct answer.
Note:
In trimethylaluminum, this Three Centre two electron bonding pattern is seen, which leads to formation of a dimer. Al2(CH3)6 involving bridges with the carbon atoms of two of the methyl groups . This type of bond also occurs in carbon compounds, which is referred to as hyperconjugation.
