Question
Question: Thermodynamic processes are indicated in the following diagram. Match the following: Column-I|...
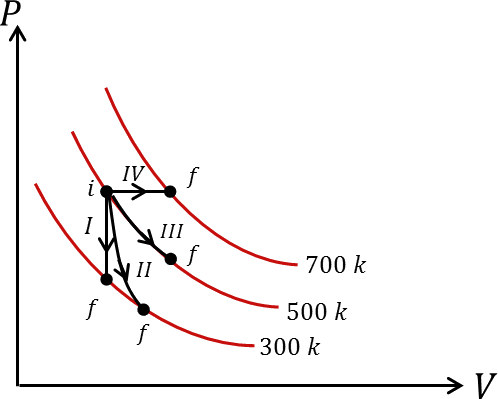
Thermodynamic processes are indicated in the following diagram.
Match the following:
| Column-I | Column-II |
|---|---|
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
A. P-d, Q-B, R-a, S-c
B. P-a, Q-c, R-d, S-b
C. P-c, Q-a, R-d, S-b
D. P-c, Q-d, R-b, S-a
Solution
Adiabatic process is a process in which there is no transfer of heat between the surrounding and the system. Isobaric process is a process in which the pressure remains constant. Isochoric process is a process in which the volume remains constant. Isothermal process is a process in which the temperature of the system remains constant.
Complete answer:
In the given diagram, we can understand the process I depicted constant volume which means it is an Isochoric Process.
Process III shows constant temperature which means it is an Isothermal Process.
Process IV shows constant pressure which means it is an Isobaric Process.
The only option which matches the above explanation is option C which tells that the process II is an Adiabatic Process.
Thus, Process I is Isochoric, Process II is Adiabatic, Process III is Isothermal and Process IV is Isobaric.
So, the correct answer is option C i.e. P-c, Q-a, R-d, S-b.
Note:
To answer these types of questions, students must have the concepts and applications of thermodynamics clear. Similarly, other curves such as Pressure vs Temperature which depicts variation in pressure and temperature, and Volume vs Temperature which depicts the variation in volume and temperature can also be asked.
